DNA methylation directs functional maturation of pancreatic β cells
- PMID: 26098213
- PMCID: PMC4563682
- DOI: 10.1172/JCI79956
DNA methylation directs functional maturation of pancreatic β cells
Abstract
Pancreatic β cells secrete insulin in response to postprandial increases in glucose levels to prevent hyperglycemia and inhibit insulin secretion under fasting conditions to protect against hypoglycemia. β cells lack this functional capability at birth and acquire glucose-stimulated insulin secretion (GSIS) during neonatal life. Here, we have shown that during postnatal life, the de novo DNA methyltransferase DNMT3A initiates a metabolic program by repressing key genes, thereby enabling the coupling of insulin secretion to glucose levels. In a murine model, β cell-specific deletion of Dnmt3a prevented the metabolic switch, resulting in loss of GSIS. DNMT3A bound to the promoters of the genes encoding hexokinase 1 (HK1) and lactate dehydrogenase A (LDHA) - both of which regulate the metabolic switch - and knockdown of these two key DNMT3A targets restored the GSIS response in islets from animals with β cell-specific Dnmt3a deletion. Furthermore, DNA methylation-mediated repression of glucose-secretion decoupling genes to modulate GSIS was conserved in human β cells. Together, our results reveal a role for DNA methylation to direct the acquisition of pancreatic β cell function.
Figures

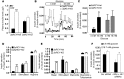
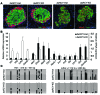
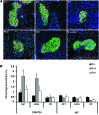
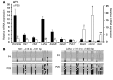
Comment in
-
Epigenetic programming of glucose-regulated insulin release.J Clin Invest. 2015 Jul 1;125(7):2565-8. doi: 10.1172/JCI82575. Epub 2015 Jun 22. J Clin Invest. 2015. PMID: 26098206 Free PMC article.
References
-
- Hedeskov CJ. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980;60(2):442–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

