Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition
- PMID: 26098214
- PMCID: PMC4563678
- DOI: 10.1172/JCI78544
Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition
Abstract
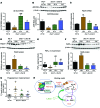
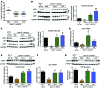
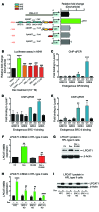
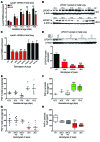
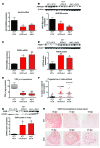
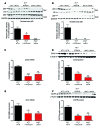
The precise mechanisms that lead to parturition are incompletely defined. Surfactant protein-A (SP-A), which is secreted by fetal lungs into amniotic fluid (AF) near term, likely provides a signal for parturition; however, SP-A-deficient mice have only a relatively modest delay (~12 hours) in parturition, suggesting additional factors. Here, we evaluated the contribution of steroid receptor coactivators 1 and 2 (SRC-1 and SRC-2), which upregulate SP-A transcription, to the parturition process. As mice lacking both SRC-1 and SRC-2 die at birth due to respiratory distress, we crossed double-heterozygous males and females. Parturition was severely delayed (~38 hours) in heterozygous dams harboring SRC-1/-2-deficient embryos. These mothers exhibited decreased myometrial NF-κB activation, PGF2α, and expression of contraction-associated genes; impaired luteolysis; and elevated circulating progesterone. These manifestations also occurred in WT females bearing SRC-1/-2 double-deficient embryos, indicating that a fetal-specific defect delayed labor. SP-A, as well as the enzyme lysophosphatidylcholine acyltransferase-1 (LPCAT1), required for synthesis of surfactant dipalmitoylphosphatidylcholine, and the proinflammatory glycerophospholipid platelet-activating factor (PAF) were markedly reduced in SRC-1/-2-deficient fetal lungs near term. Injection of PAF or SP-A into AF at 17.5 days post coitum enhanced uterine NF-κB activation and contractile gene expression, promoted luteolysis, and rescued delayed parturition in SRC-1/-2-deficient embryo-bearing dams. These findings reveal that fetal lungs produce signals to initiate labor when mature and that SRC-1/-2-dependent production of SP-A and PAF is crucial for this process.
Figures

Comment in
-
Fetal-to-maternal signaling to initiate parturition.J Clin Invest. 2015 Jul 1;125(7):2569-71. doi: 10.1172/JCI82576. Epub 2015 Jun 22. J Clin Invest. 2015. PMID: 26098207 Free PMC article.
-
Pregnancy: Fetal signalling initiates parturition.Nat Rev Endocrinol. 2015 Sep;11(9):505. doi: 10.1038/nrendo.2015.115. Epub 2015 Jul 7. Nat Rev Endocrinol. 2015. PMID: 26149616 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

