Synaptic pathology and therapeutic repair in adult retinoschisis mouse by AAV-RS1 transfer
- PMID: 26098217
- PMCID: PMC4563692
- DOI: 10.1172/JCI81380
Synaptic pathology and therapeutic repair in adult retinoschisis mouse by AAV-RS1 transfer
Abstract
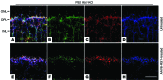
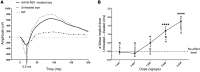
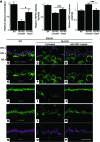
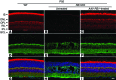
Strategies aimed at invoking synaptic plasticity have therapeutic potential for several neurological conditions. The human retinal synaptic disease X-linked retinoschisis (XLRS) is characterized by impaired visual signal transmission through the retina and progressive visual acuity loss, and mice lacking retinoschisin (RS1) recapitulate human disease. Here, we demonstrate that restoration of RS1 via retina-specific delivery of adeno-associated virus type 8-RS1 (AAV8-RS1) vector rescues molecular pathology at the photoreceptor-depolarizing bipolar cell (photoreceptor-DBC) synapse and restores function in adult Rs1-KO animals. Initial development of the photoreceptor-DBC synapse was normal in the Rs1-KO retina; however, the metabotropic glutamate receptor 6/transient receptor potential melastatin subfamily M member 1-signaling (mGluR6/TRPM1-signaling) cascade was not properly maintained. Specifically, the TRPM1 channel and G proteins Gαo, Gβ5, and RGS11 were progressively lost from postsynaptic DBC dendritic tips, whereas the mGluR6 receptor and RGS7 maintained proper synaptic position. This postsynaptic disruption differed from other murine night-blindness models with an electronegative electroretinogram response, which is also characteristic of murine and human XLRS disease. Upon AAV8-RS1 gene transfer to the retina of adult XLRS mice, TRPM1 and the signaling molecules returned to their proper dendritic tip location, and the DBC resting membrane potential was restored. These findings provide insight into the molecular plasticity of a critical synapse in the visual system and demonstrate potential therapeutic avenues for some diseases involving synaptic pathology.
Figures
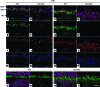
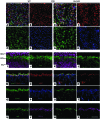
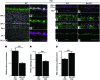
Comment in
-
Restoration of synaptic function in sight for degenerative retinal disease.J Clin Invest. 2015 Jul 1;125(7):2572-5. doi: 10.1172/JCI82577. Epub 2015 Jun 22. J Clin Invest. 2015. PMID: 26098210 Free PMC article.
References
-
- Jarraya B, et al. Dopamine gene therapy for Parkinson’s disease in a nonhuman primate without associated dyskinesia. Sci Transl Med. 2009;1(2):2ra4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

