Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env
- PMID: 26098315
- PMCID: PMC4706170
- DOI: 10.1038/nsmb.3051
Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env
Abstract
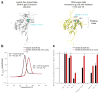
As the sole viral antigen on the HIV-1-virion surface, trimeric Env is a focus of vaccine efforts. Here we present the structure of the ligand-free HIV-1-Env trimer, fix its conformation and determine its receptor interactions. Epitope analyses revealed trimeric ligand-free Env to be structurally compatible with broadly neutralizing antibodies but not poorly neutralizing ones. We coupled these compatibility considerations with binding antigenicity to engineer conformationally fixed Envs, including a 201C 433C (DS) variant specifically recognized by broadly neutralizing antibodies. DS-Env retained nanomolar affinity for the CD4 receptor, with which it formed an asymmetric intermediate: a closed trimer bound by a single CD4 without the typical antigenic hallmarks of CD4 induction. Antigenicity-guided structural design can thus be used both to delineate mechanism and to fix conformation, with DS-Env trimers in virus-like-particle and soluble formats providing a new generation of vaccine antigens.
Conflict of interest statement
Figures




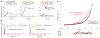


References
-
- Starcich BR, et al. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986;45:637–48. - PubMed
-
- Wei X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. - PubMed
-
- Kwong PD, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01-AI93278/AI/NIAID NIH HHS/United States
- P01-HL59725/HL/NHLBI NIH HHS/United States
- P01-AI104722/AI/NIAID NIH HHS/United States
- P01 AI100151/AI/NIAID NIH HHS/United States
- R01-GM78031/GM/NIGMS NIH HHS/United States
- R01-GM98859/GM/NIGMS NIH HHS/United States
- R21-AI112389/AI/NIAID NIH HHS/United States
- ZIA AI005024/ImNIH/Intramural NIH HHS/United States
- R33-AI84714/AI/NIAID NIH HHS/United States
- R01 GM098859/GM/NIGMS NIH HHS/United States
- R21 AI112389/AI/NIAID NIH HHS/United States
- P01 GM056550/GM/NIGMS NIH HHS/United States
- P01-AI100151/AI/NIAID NIH HHS/United States
- R01 HL059725/HL/NHLBI NIH HHS/United States
- R21 AI100696/AI/NIAID NIH HHS/United States
- HHSN261200800001E./PHS HHS/United States
- R01 GM078031/GM/NIGMS NIH HHS/United States
- P01-GM56550/GM/NIGMS NIH HHS/United States
- R01 AI100790/AI/NIAID NIH HHS/United States
- R33 AI084714/AI/NIAID NIH HHS/United States
- R21-AI100696/AI/NIAID NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- R01 AI093278/AI/NIAID NIH HHS/United States
- P01 AI104722/AI/NIAID NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

