Monosynaptic convergence of somatic and visceral C-fiber afferents on projection and local circuit neurons in lamina I: a substrate for referred pain
- PMID: 26098437
- PMCID: PMC4770360
- DOI: 10.1097/j.pain.0000000000000267
Monosynaptic convergence of somatic and visceral C-fiber afferents on projection and local circuit neurons in lamina I: a substrate for referred pain
Abstract
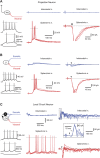
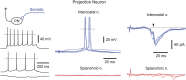
Referred pain is a phenomenon of feeling pain at a site other than the site of the painful stimulus origin. It arises from a pathological mixing of nociceptive processing pathways for visceral and somatic inputs. Despite numerous studies based on unit recordings from spinal and supraspinal neurons, the exact mechanism and site of this mixing within the central nervous system are not known. Here, we selectively recorded from lamina I neurons, using a visually guided patch-clamp technique, in thoracic spinal cord preparation with preserved intercostal (somatic) and splanchnic (visceral) nerves. We show that somatic and visceral C fibers converge monosynaptically onto a group of lamina I neurons, which includes both projection and local circuit neurons. Other groups of lamina I neurons received inputs from either somatic or visceral afferents. We have also identified a population of lamina I local circuit neurons showing overall inhibitory responses upon stimulation of both nerves. Thus, the present data allow us to draw two major conclusions. First, lamina I of the spinal cord is the first site in the central nervous system where somatic and visceral pathways directly converge onto individual projection and local circuit neurons. Second, the mechanism of somatovisceral convergence is complex and based on functional integration of monosynaptic and polysynaptic excitatory as well as inhibitory inputs in specific groups of neurons. This complex pattern of convergence provides a substrate for alterations in the balance between visceral and somatic inputs causing referred pain.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures




References
-
- Akeyson EW, Schramm LP. Processing of splanchnic and somatic input in thoracic spinal cord of the rat. Am J Physiol 1994;266:R257–267. - PubMed
-
- Akeyson EW, Schramm LP. Splanchnic and somatic afferent convergence on cervical spinal neurons of the rat. Am J Physiol 1994;266:R268–276. - PubMed
-
- Alarcon G, Cervero F. The effects of electrical stimulation of A and C visceral afferent fibres on the excitability of viscerosomatic neurones in the thoracic spinal cord of the cat. Brain Res 1990;509:24–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

