Lineage conversion induced by pluripotency factors involves transient passage through an iPSC stage
- PMID: 26098450
- PMCID: PMC4840929
- DOI: 10.1038/nbt.3247
Lineage conversion induced by pluripotency factors involves transient passage through an iPSC stage
Abstract
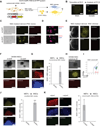
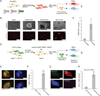
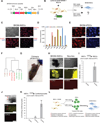
Brief expression of pluripotency-associated factors such as Oct4, Klf4, Sox2 and c-Myc (OKSM), in combination with differentiation-inducing signals, has been reported to trigger transdifferentiation of fibroblasts into other cell types. Here we show that OKSM expression in mouse fibroblasts gives rise to both induced pluripotent stem cells (iPSCs) and induced neural stem cells (iNSCs) under conditions previously shown to induce only iNSCs. Fibroblast-derived iNSC colonies silenced retroviral transgenes and reactivated silenced X chromosomes, both hallmarks of pluripotent stem cells. Moreover, lineage tracing with an Oct4-CreER labeling system demonstrated that virtually all iNSC colonies originated from cells transiently expressing Oct4, whereas ablation of Oct4(+) cells prevented iNSC formation. Lastly, an alternative transdifferentiation cocktail that lacks Oct4 and was reportedly unable to support induced pluripotency yielded iPSCs and iNSCs carrying the Oct4-CreER-derived lineage label. Together, these data suggest that iNSC generation from fibroblasts using OKSM and other pluripotency-related reprogramming factors requires passage through a transient iPSC state.
Figures

Comment in
-
Transdifferentiation from the top.Nat Methods. 2015 Aug;12(8):701. doi: 10.1038/nmeth.3509. Nat Methods. 2015. PMID: 26451422 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

