Coadministration of Hedera helix L. Extract Enabled Mice to Overcome Insufficient Protection against Influenza A/PR/8 Virus Infection under Suboptimal Treatment with Oseltamivir
- PMID: 26098681
- PMCID: PMC4476699
- DOI: 10.1371/journal.pone.0131089
Coadministration of Hedera helix L. Extract Enabled Mice to Overcome Insufficient Protection against Influenza A/PR/8 Virus Infection under Suboptimal Treatment with Oseltamivir
Abstract
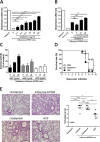
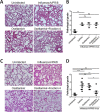
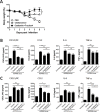
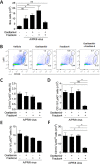
Several anti-influenza drugs that reduce disease manifestation exist, and although these drugs provide clinical benefits in infected patients, their efficacy is limited by the emergence of drug-resistant influenza viruses. In the current study, we assessed the therapeutic strategy of enhancing the antiviral efficacy of an existing neuraminidase inhibitor, oseltamivir, by coadministering with the leaf extract from Hedera helix L, commonly known as ivy. Ivy extract has anti-inflammatory, antibacterial, antifungal, and antihelminthic properties. In the present study, we investigated its potential antiviral properties against influenza A/PR/8 (PR8) virus in a mouse model with suboptimal oseltamivir that mimics a poor clinical response to antiviral drug treatment. Suboptimal oseltamivir resulted in insufficient protection against PR8 infection. Oral administration of ivy extract with suboptimal oseltamivir increased the antiviral activity of oseltamivir. Ivy extract and its compounds, particularly hedrasaponin F, significantly reduced the cytopathic effect in PR8-infected A549 cells in the presence of oseltamivir. Compared with oseltamivir treatment alone, coadministration of the fraction of ivy extract that contained the highest proportion of hedrasaponin F with oseltamivir decreased pulmonary inflammation in PR8-infected mice. Inflammatory cytokines and chemokines, including tumor necrosis factor-alpha and chemokine (C-C motif) ligand 2, were reduced by treatment with oseltamivir and the fraction of ivy extract. Analysis of inflammatory cell infiltration in the bronchial alveolar of PR8-infected mice revealed that CD11b+Ly6G+ and CD11b+Ly6Cint cells were recruited after virus infection; coadministration of the ivy extract fraction with oseltamivir reduced infiltration of these inflammatory cells. In a model of suboptimal oseltamivir treatment, coadministration of ivy extract fraction that includes hedrasaponin F increased protection against PR8 infection that could be explained by its antiviral and anti-inflammatory activities.
Conflict of interest statement
Figures


References
-
- Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12(2–3):171–80. Epub 2001/04/28. . - PubMed
-
- Julkunen I, Melen K, Nyqvist M, Pirhonen J, Sareneva T, Matikainen S. Inflammatory responses in influenza A virus infection. Vaccine. 2000;19 Suppl 1:S32–7. Epub 2001/02/13. . - PubMed
-
- Monto AS, McKimm-Breschkin JL, Macken C, Hampson AW, Hay A, Klimov A, et al. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother. 2006;50(7):2395–402. Epub 2006/06/28. 10.1128/aac.01339-05 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

