Activation of PI3K/Akt/mTOR signaling in the tumor stroma drives endocrine therapy-dependent breast tumor regression
- PMID: 26098779
- PMCID: PMC4673148
- DOI: 10.18632/oncotarget.4203
Activation of PI3K/Akt/mTOR signaling in the tumor stroma drives endocrine therapy-dependent breast tumor regression
Erratum in
-
Correction: Activation of PI3K/Akt/mTOR signaling in the tumor stroma drives endocrine therapy-dependent breast tumor regression.Oncotarget. 2025 May 20;16:379-381. doi: 10.18632/oncotarget.28728. Oncotarget. 2025. PMID: 40391748 Free PMC article. No abstract available.
Abstract
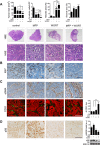
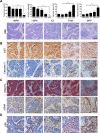
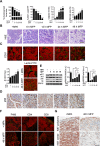
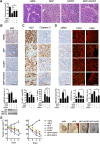
Improved efficacy of neoadjuvant endocrine-targeting therapies in luminal breast carcinomas could be achieved with optimal use of pathway targeting agents. In a mouse model of ductal breast carcinoma we identify a tumor regressive stromal reaction that is induced by neoadjuvant endocrine therapy. This reparative reaction is characterized by tumor neovascularization accompanied by infiltration of immune cells and carcinoma-associated fibroblasts that stain for phosphorylated ribosomal protein S6 (pS6), downstream the PI3K/Akt/mTOR pathway. While tumor variants with higher PI3K/Akt/mTOR activity respond well to a combination of endocrine and PI3K/Akt/mTOR inhibitors, tumor variants with lower PI3K/Akt/mTOR activity respond more poorly to the combination therapy than to the endocrine therapy alone, associated with inhibition of stromal pS6 and the reparative reaction. In human breast cancer xenografts we confirm that such differential sensitivity to therapy is primarily determined by the level of PI3K/Akt/mTOR in tumor cells. We further show that the clinical response of breast cancer patients undergoing neoadjuvant endocrine therapy is associated with the reparative stromal reaction. We conclude that tumor level and localization of pS6 are associated with therapeutic response in breast cancer and represent biomarkers to distinguish which tumors will benefit from the incorporation of PI3K/Akt/mTOR inhibitors with neoadjuvant endocrine therapy.
Keywords: PI3K/Akt pathway; breast cancer; neoadjuvant endocrine therapy; tumor stroma.
Conflict of interest statement
The authors have no competing interests to declare.
Figures


References
-
- Charehbili A, Fontein DB, Kroep JR, Liefers GJ, Mieog JS, Nortier JW, et al. Neoadjuvant hormonal therapy for endocrine sensitive breast cancer: a systematic review. Cancer Treat.Rev. 2014;40:86–92. - PubMed
-
- Lange CA, Sartorius CA, Abdel-Hafiz H, Spillman MA, Horwitz KB, Jacobsen BM. Progesterone receptor action: translating studies in breast cancer models to clinical insights. Adv.Exp.Med.Biol. 2008;630:94–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

