Predictors of Pregnancy Outcomes in Patients With Lupus: A Cohort Study
- PMID: 26098843
- PMCID: PMC5113288
- DOI: 10.7326/M14-2235
Predictors of Pregnancy Outcomes in Patients With Lupus: A Cohort Study
Abstract
Background: Because systemic lupus erythematosus (SLE) affects women of reproductive age, pregnancy is a major concern.
Objective: To identify predictors of adverse pregnancy outcomes (APOs) in patients with inactive or stable active SLE.
Design: Prospective cohort.
Setting: Multicenter.
Patients: 385 patients (49% non-Hispanic white; 31% with prior nephritis) with SLE in the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study. Exclusion criteria were urinary protein-creatinine ratio greater than 1000 mg/g, creatinine level greater than 1.2 mg/dL, prednisone use greater than 20 mg/d, and multifetal pregnancy.
Measurements: APOs included fetal or neonatal death; birth before 36 weeks due to placental insufficiency, hypertension, or preeclampsia; and small-for-gestational-age (SGA) neonate (birthweight below the fifth percentile). Disease activity was assessed with the Systemic Lupus Erythematosus Pregnancy Disease Activity Index and the Physician's Global Assessment (PGA).
Results: APOs occurred in 19.0% (95% CI, 15.2% to 23.2%) of pregnancies; fetal death occurred in 4%, neonatal death occurred in 1%, preterm delivery occurred in 9%, and SGA neonate occurred in 10%. Severe flares in the second and third trimesters occurred in 2.5% and 3.0%, respectively. Baseline predictors of APOs included presence of lupus anticoagulant (LAC) (odds ratio [OR], 8.32 [CI, 3.59 to 19.26]), antihypertensive use (OR, 7.05 [CI, 3.05 to 16.31]), PGA score greater than 1 (OR, 4.02 [CI, 1.84 to 8.82]), and low platelet count (OR, 1.33 [CI, 1.09 to 1.63] per decrease of 50 × 109 cells/L). Non-Hispanic white race was protective (OR, 0.45 [CI, 0.24 to 0.84]). Maternal flares, higher disease activity, and smaller increases in C3 level later in pregnancy also predicted APOs. Among women without baseline risk factors, the APO rate was 7.8%. For those who either were LAC-positive or were LAC-negative but nonwhite or Hispanic and using antihypertensives, the APO rate was 58.0% and fetal or neonatal mortality was 22.0%.
Limitation: Patients with high disease activity were excluded.
Conclusion: In pregnant patients with inactive or stable mild/moderate SLE, severe flares are infrequent and, absent specific risk factors, outcomes are favorable.
Primary funding source: National Institutes of Health.
Figures
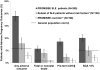
Comment in
-
Pregnancy in Women With Systemic Lupus Erythematosus: Messages for the Clinician.Ann Intern Med. 2015 Aug 4;163(3):232-3. doi: 10.7326/M15-1301. Ann Intern Med. 2015. PMID: 26099038 No abstract available.
-
Predictors of Pregnancy Outcomes in Patients With Lupus.Ann Intern Med. 2016 Jan 19;164(2):131. doi: 10.7326/L15-0499. Ann Intern Med. 2016. PMID: 26784478 No abstract available.
-
Predictors of Pregnancy Outcomes in Patients With Lupus.Ann Intern Med. 2016 Jan 19;164(2):131. doi: 10.7326/L15-0500. Ann Intern Med. 2016. PMID: 26784479 No abstract available.
-
Pregnancies in women with systemic lupus erythematosus and antiphospholipid antibodies.Lupus. 2016 Apr;25(4):343-5. doi: 10.1177/0961203315627201. Epub 2016 Jan 24. Lupus. 2016. PMID: 26811370
Summary for patients in
-
Summaries for Patients. Pregnancy Outcomes in Patients With Lupus.Ann Intern Med. 2015 Aug 4;163(3):I-23. doi: 10.7326/P15-9024. Ann Intern Med. 2015. PMID: 26098964 No abstract available.
References
-
- Silva CA, Leal MM, Leone C, Simone VP, Takiuti AD, Saito MI, et al. Gonadal function in adolescents and young women with juvenile systemic lupus erythematosus. Lupus. 2002;11(7):419–25. - PubMed
-
- Ostensen M. New insights into sexual functioning and fertility in rheumatic diseases. Best Pract Res Clin Rheumatol. 2004;18(2):219–32. - PubMed
-
- Petri M, Allbritton J. Fetal outcome of lupus pregnancy: a retrospective case-control study of the Hopkins Lupus Cohort. J Rheumatol. 1993;20(4):650–6. - PubMed
-
- Georgiou PE, Politi EN, Katsimbri P, Sakka V, Drosos AA. Outcome of lupus pregnancy: a controlled study. Rheumatology (Oxford) 2000;39(9):1014–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
