Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study
- PMID: 26099227
- PMCID: PMC4477007
- DOI: 10.1186/s13550-015-0114-2
Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study
Abstract
Background: Radioligand therapy (RLT) with (177)Lu-DKFZ-617 PSMA (Lu-PSMA) (prostate-specific membrane antigen) is a novel targeted therapy of metastatic prostate cancer. We analysed retrospectively the early side effects and the response rate in the first patients, who received a therapy with Lu-PSMA in our departments.
Methods: RLT was performed in ten hormone- and/or chemo-refractory patients with distant metastases and progressive disease (mean age 73.5 years). (68)Ga-PSMA HBED-CC PET/CT was performed in all patients prior to RLT. The median PSA level prior to the therapy was 298.5 ng/ml (range 5-853 ng/ml). All patients received CBC, renal and liver function tests the day before and 2 days after application (mean administered activity 5.6 GBq, range 4.1-6.1 GBq), followed by further tests every 2 weeks. All patients were contacted by telephone every week regarding side effects or any positive and negative changes.
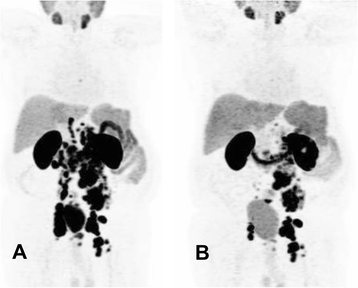
Results: Eight weeks after the therapy, seven patients (70 %) experienced a PSA decline, of whom six experienced more than 30 % and five more than 50 %. Three patients showed a progressive disease according to the PSA increase. No patient experienced any side effects immediately after injection of Lu-PSMA. Relevant hematotoxicity (grade 3 or 4) occurred 7 weeks after the administration in just one patient. The same patient showed a leucopenia grade 2. Two patients showed a disturbance of only 1 hematologic cell line, whereas one patient showed a reduction of grades 1 and 2 in leucocytes and thrombocytes, respectively. Six patients did not show any hematotoxicity during the 8 weeks after therapy. There was no relevant nephrotoxicity (grade 3 or 4).
Conclusions: Our initial results indicate that RLT with Lu-PSMA is safe and seems to have low early side-effect profile. A relevant PSA decline was detected in 70 % of patients.
Figures
References
-
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–60. doi: 10.1016/S1470-2045(14)71205-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

