Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters
- PMID: 26099350
- PMCID: PMC4762928
- DOI: 10.1007/s00109-015-1307-x
Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters
Abstract
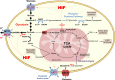
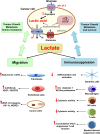
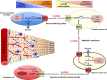
Since Otto Warburg reported the 'addiction' of cancer cells to fermentative glycolysis, a metabolic pathway that provides energy and building blocks, thousands of studies have shed new light on the molecular mechanisms contributing to altered cancer metabolism. Hypoxia, through hypoxia-inducible factors (HIFs), in addition to oncogenes activation and loss of tumour suppressors constitute major regulators of not only the "Warburg effect" but also many other metabolic pathways such as glutaminolysis. Enhanced glucose and glutamine catabolism has become a recognised feature of cancer cells, leading to accumulation of metabolites in the tumour microenvironment, which offers growth advantages to tumours. Among these metabolites, lactic acid, besides imposing an acidic stress, is emerging as a key signalling molecule that plays a pivotal role in cancer cell migration, angiogenesis, immune escape and metastasis. Although interest in lactate for cancer development only appeared recently, pharmacological molecules blocking its metabolism are already in phase I/II clinical trials. Here, we review the metabolic pathways generating lactate, and we discuss the rationale for targeting lactic acid transporter complexes for the development of efficient and selective anticancer therapies.
Keywords: BASIGIN; Cancer; Lactate; MCT; Monocarboxylate Transporters; Therapy; Warburg effect.
Figures


References
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. - PubMed
-
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

