Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials
- PMID: 26099511
- PMCID: PMC4477483
- DOI: 10.1186/s12916-015-0358-8
Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials
Abstract
Background: Inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) has been intensively studied to lower low-density lipoprotein cholesterol (LDL-C) levels. The purpose of this meta-analysis was to evaluate the safety and efficacy of anti-PCSK9 antibodies in randomized, controlled trials (RCTs).
Methods: PubMed, EMBASE, CENTRAL databases, and recent conferences were searched. Safety outcomes were rates of common adverse events. Efficacy outcomes included percentages of LDL-C lowering and other lipid changes compared with placebo and ezetimibe, respectively.
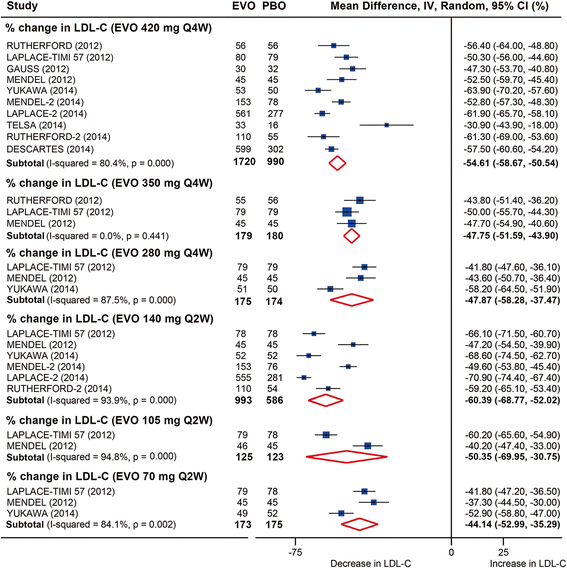
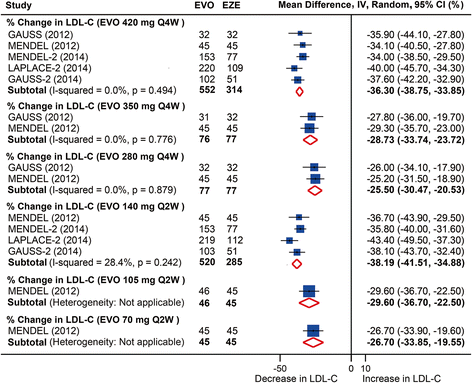
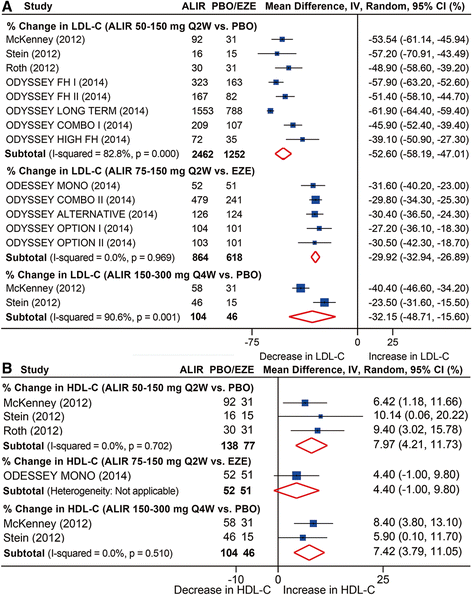
Results: Twenty-five RCTs encompassing 12,200 patients were included. The rates of common adverse events were firstly reported in our study by pooling together all evidence in RCTs, showing largely no significant difference between anti-PCSK9 antibodies and placebo (or ezetimibe), except that alirocumab was associated with reduced rates of death (relative risk (RR): 0.43, 95 % confidence interval (CI): 0.19 to 0.96, P = 0.04) and an increased rate of injection-site reactions (RR: 1.48, 95 % CI: 1.05 to 2.09, P = 0.02); evolocumab reduced the rate of abnormal liver function (RR: 0.43, 95 % CI: 0.20 to 0.93, P = 0.03), both compared with placebo. No significant difference in safety outcomes was detected between monthly 420 mg and biweekly 140 mg evolocumab treatments. Monthly 420 mg evolocumab treatment significantly reduced LDL-C by -54.6 % (95 % CI: -58.7 to -50.5 %) and by absolute -78.9 mg/dl (95 % CI: -88.9 to -68.9 mg/dl) versus placebo, and by -36.3 % (95 % CI: -38.8 to -33.9 %) versus ezetimibe, and increased high-density lipoprotein cholesterol (HDL-C) by 7.6 % (95 % CI: 5.7 to 9.5 %) versus placebo and 6.4 % (95 % CI: 4.3 to 8.4 %) versus ezetimibe. An equal or even greater change was observed following biweekly 140 mg administration. Significant and favorable changes were also detected in other lipids following evolocumab treatment. Biweekly 50 to 150 mg alirocumab lowered LDL-C by -52.6 % (95 % CI: -58.2 to -47.0 %) versus placebo, by -29.9 % (95 % CI: -32.9 to -26.9 %) versus ezetimibe, and increased HDL-C by 8.0 % (95 % CI: 4.2 to 11.7 %) versus placebo.
Conclusions: Evolocumab and alirocumab were safe and well-tolerated from our most-powered analyses. Both antibodies substantially reduced the LDL-C level by over 50 %, increased the HDL-C level, and resulted in favorable changes in other lipids.
Figures


References
-
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. - DOI - PubMed
-
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey MC, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. - DOI - PubMed
-
- Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–12. doi: 10.1074/jbc.M702027200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

