Expression of the Oligopeptide Permease Operon of Moraxella catarrhalis Is Regulated by Temperature and Nutrient Availability
- PMID: 26099587
- PMCID: PMC4534648
- DOI: 10.1128/IAI.00597-15
Expression of the Oligopeptide Permease Operon of Moraxella catarrhalis Is Regulated by Temperature and Nutrient Availability
Abstract
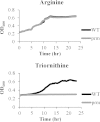
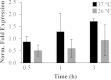
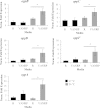
Moraxella catarrhalis causes otitis media in children and exacerbations of chronic obstructive pulmonary disease in adults. Together, these two conditions contribute to enormous morbidity and mortality worldwide. The oligopeptide permease (opp) ABC transport system is a nutritional virulence factor important for the utilization of peptides. The substrate binding protein OppA, which binds peptides for uptake, is a potential vaccine antigen, but little was known about the regulation of gene expression. The five opp genes oppB, oppC, oppD, oppF, and oppA are in the same open reading frame. Sequence analysis predicted two promoters, one located upstream of oppB and one within the intergenic region between oppF and oppA. We have characterized the gene cluster as an operon with two functional promoters and show that cold shock at 26°C for ≤ 0.5 h and the presence of a peptide substrate increase gene transcript levels. Additionally, the putative promoter upstream of oppA contributes to the transcription of oppA but is not influenced by the same environmental cues as the promoter upstream of oppB. We conclude that temperature and nutrient availability contribute to the regulation of the Opp system, which is an important nutritional virulence factor in M. catarrhalis.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






Similar articles
-
Role of the oligopeptide permease ABC Transporter of Moraxella catarrhalis in nutrient acquisition and persistence in the respiratory tract.Infect Immun. 2014 Nov;82(11):4758-66. doi: 10.1128/IAI.02185-14. Epub 2014 Aug 25. Infect Immun. 2014. PMID: 25156736 Free PMC article.
-
Functional testing of putative oligopeptide permease (Opp) proteins of Borrelia burgdorferi: a complementation model in opp(-) Escherichia coli.Biochim Biophys Acta. 2001 Jan 15;1499(3):222-31. doi: 10.1016/s0167-4889(00)00121-x. Biochim Biophys Acta. 2001. PMID: 11341969
-
Genetic characterization of an oligopeptide transport system from Lactobacillus delbrueckii subsp. bulgaricus.Arch Microbiol. 2002 Jun;177(6):457-67. doi: 10.1007/s00203-002-0411-9. Epub 2002 Mar 22. Arch Microbiol. 2002. PMID: 12029391
-
Effects of oligopeptide permease in group a streptococcal infection.Infect Immun. 2005 May;73(5):2881-90. doi: 10.1128/IAI.73.5.2881-2890.2005. Infect Immun. 2005. PMID: 15845494 Free PMC article.
-
Identification of oligopeptide-binding protein (OppA) and its role in the virulence of Streptococcus suis serotype 2.Microb Pathog. 2018 May;118:322-329. doi: 10.1016/j.micpath.2018.03.061. Epub 2018 Mar 31. Microb Pathog. 2018. PMID: 29614370
Cited by
-
Panel 8: Vaccines and immunology.Int J Pediatr Otorhinolaryngol. 2020 Mar;130 Suppl 1(Suppl 1):109839. doi: 10.1016/j.ijporl.2019.109839. Epub 2019 Dec 18. Int J Pediatr Otorhinolaryngol. 2020. PMID: 31948716 Free PMC article. Review.
-
The impact of environmental factors on respiratory tract microbiome and respiratory system diseases.Eur J Med Res. 2025 Apr 4;30(1):236. doi: 10.1186/s40001-025-02517-3. Eur J Med Res. 2025. PMID: 40186246 Free PMC article. Review.
-
Adaptation of Nontypeable Haemophilus influenzae in Human Airways in COPD: Genome Rearrangements and Modulation of Expression of HMW1 and HMW2.mBio. 2023 Apr 25;14(2):e0014023. doi: 10.1128/mbio.00140-23. Epub 2023 Mar 16. mBio. 2023. PMID: 36927061 Free PMC article.
-
Toxicity and inhibition mechanism of gallic acid on physiology and fermentation performance of Escherichia coli.Bioresour Bioprocess. 2022 Jul 23;9(1):76. doi: 10.1186/s40643-022-00564-w. Bioresour Bioprocess. 2022. PMID: 38647760 Free PMC article.
-
Potential impact of a Moraxella catarrhalis vaccine in COPD.Vaccine. 2019 Sep 3;37(37):5551-5558. doi: 10.1016/j.vaccine.2016.12.066. Epub 2017 Feb 6. Vaccine. 2019. PMID: 28185742 Free PMC article. Review.
References
-
- Vergison A, Dagan R, Arguedas A, Bonhoeffer J, Cohen R, Dhooge I, Hoberman A, Liese J, Marchisio P, Palmu AA, Ray GT, Sanders EA, Simoes EA, Uhari M, van Eldere J, Pelton SI. 2010. Otitis media and its consequences: beyond the earache. Lancet Infect Dis 10:195–203. doi:10.1016/S1473-3099(10)70012-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

