Dinitrogen binding and cleavage by multinuclear iron complexes
- PMID: 26099821
- PMCID: PMC4819597
- DOI: 10.1021/acs.accounts.5b00213
Dinitrogen binding and cleavage by multinuclear iron complexes
Abstract
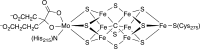
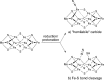
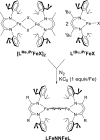
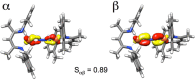
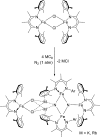
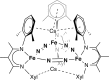
The iron-molybdenum cofactor of nitrogenase has unprecedented coordination chemistry, including a high-spin iron cluster called the iron-molybdenum cofactor (FeMoco). Thus, understanding the mechanism of nitrogenase challenges coordination chemists to understand the fundamental N2 chemistry of high-spin iron sites. This Account summarizes a series of studies in which we have synthesized a number of new compounds with multiple iron atoms, characterized them using crystallography and spectroscopy, and studied their reactions in detail. These studies show that formally iron(I) and iron(0) complexes with three- and four-coordinate metal atoms have the ability to weaken and break the triple bond of N2. These reactions occur at or below room temperature, indicating that they are kinetically facile. This in turn implies that iron sites in the FeMoco are chemically reasonable locations for N2 binding and reduction. The careful evaluation of these compounds and their reaction pathways has taught important lessons about what characteristics make iron more effective for N2 activation. Cooperation of two iron atoms can lengthen and weaken the N-N bond, while three working together enables iron atoms to completely cleave the N-N bond to nitrides. Alkali metals (typically introduced into the reaction as part of the reducing agent) are thermodynamically useful because the alkali metal cations stabilize highly reduced complexes, pull electron density into the N2 unit, and make reduced nitride products more stable. Alkali metals can also play a kinetic role, because cation-π interactions with the supporting ligands can hold iron atoms near enough to one another to facilitate the cooperation of multiple iron atoms. Many of these principles may also be relevant to the iron-catalyzed Haber-Bosch process, at which collections of iron atoms (often promoted by the addition of alkali metals) break the N-N bond of N2. The results of these studies teach more general lessons as well. They have demonstrated that N2 can be a redox-active ligand, accepting spin and electron density in complexes of N2(2-). They have shown the power of cooperation between multiple transition metals, and also between alkali metals and transition metals. Finally, alkali metal based cation-π interactions have the potential to be broadly useful for bringing metals close together with sufficient flexibility to allow multistep, multielectron reactions. At the same time, the positive charge on the alkali metal cation stabilizes charge buildup in intermediates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

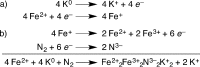

References
-
- Burgess B. K.; Lowe D. J. Mechanism of Molybdenum Nitrogenase. Chem. Rev. 1996, 96, 2983–3012. - PubMed
-
- Bjornsson R.; Lima F. A.; Spatzal T.; Weyhermuller T.; Glatzel P.; Bill E.; Einsle O.; Neese F.; DeBeer S. Identification of a spin-coupled Mo(III) in the nitrogenase iron-molybdenum cofactor. Chem. Sci. 2014, 5, 3096–3103.
-
- Dos Santos P. C.; Igarashi R. Y.; Lee H.-I.; Hoffman B. M.; Seefeldt L. C.; Dean D. R. Substrate interactions with the nitrogenase active site. Acc. Chem. Res. 2005, 38, 208–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

