Pancreatic duct glands (PDGs) are a progenitor compartment responsible for pancreatic ductal epithelial repair
- PMID: 26100232
- PMCID: PMC5396061
- DOI: 10.1016/j.scr.2015.05.006
Pancreatic duct glands (PDGs) are a progenitor compartment responsible for pancreatic ductal epithelial repair
Abstract
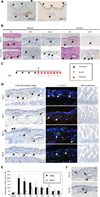
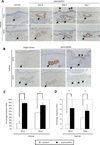
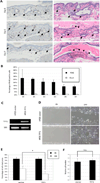
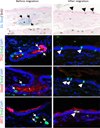
Pancreatic duct glands (PDGs) have molecular features known to mark stem cell niches, but their function remains to be determined. To investigate the role of PDGs as a progenitor niche, PDGs were analyzed in both humans and mice. Cells were characterized by immunohistochemistry and microarray analysis. In vivo proliferative activity and migration of PDG cells were evaluated using a BrdU tag-and-chase strategy in a mouse model of pancreatitis. In vitro migration assays were used to determine the role of trefoil factor (TFF) -1 and 2 in cell migration. Proliferative activity in the pancreatic epithelium in response to inflammatory injury is identified principally within the PDG compartment. These proliferating cells then migrate out of the PDG compartment to populate the pancreatic duct. Most of the pancreatic epithelial migration occurs within 5days and relies, in part, on TFF-1 and -2. After migration, PDG cells lose their PDG-specific markers and gain a more mature pancreatic ductal phenotype. Expression analysis of the PDG epithelium reveals enrichment of embryonic and stem cell pathways. These results suggest that PDGs are an epithelial progenitor compartment that gives rise to mature differentiated progeny that migrate to the pancreatic duct. Thus PDGs are a progenitor niche important for pancreatic epithelial regeneration.
Copyright © 2015. Published by Elsevier B.V.
Conflict of interest statement
There is no conflict of interest.
Figures


Similar articles
-
Progenitor cell niches in the human pancreatic duct system and associated pancreatic duct glands: an anatomical and immunophenotyping study.J Anat. 2016 Mar;228(3):474-86. doi: 10.1111/joa.12418. Epub 2015 Nov 27. J Anat. 2016. PMID: 26610370 Free PMC article.
-
Loss of Trefoil Factor 2 From Pancreatic Duct Glands Promotes Formation of Intraductal Papillary Mucinous Neoplasms in Mice.Gastroenterology. 2016 Dec;151(6):1232-1244.e10. doi: 10.1053/j.gastro.2016.07.045. Epub 2016 Aug 12. Gastroenterology. 2016. PMID: 27523981 Free PMC article.
-
In the setting of β-cell stress, the pancreatic duct gland transcriptome shows characteristics of an activated regenerative response.Am J Physiol Gastrointest Liver Physiol. 2018 Nov 1;315(5):G848-G854. doi: 10.1152/ajpgi.00177.2018. Epub 2018 Aug 10. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 30095296 Free PMC article.
-
The pancreatic ductal epithelium serves as a potential pool of progenitor cells.Pediatr Diabetes. 2004;5 Suppl 2:16-22. doi: 10.1111/j.1399-543X.2004.00075.x. Pediatr Diabetes. 2004. PMID: 15601370 Review.
-
Progenitor Epithelium: Sorting Out Pancreatic Lineages.J Histochem Cytochem. 2015 Aug;63(8):559-74. doi: 10.1369/0022155415586441. J Histochem Cytochem. 2015. PMID: 26216134 Free PMC article. Review.
Cited by
-
P2RY1/ALK3-Expressing Cells within the Adult Human Exocrine Pancreas Are BMP-7 Expandable and Exhibit Progenitor-like Characteristics.Cell Rep. 2018 Feb 27;22(9):2408-2420. doi: 10.1016/j.celrep.2018.02.006. Cell Rep. 2018. PMID: 29490276 Free PMC article.
-
Progenitor cell niches in the human pancreatic duct system and associated pancreatic duct glands: an anatomical and immunophenotyping study.J Anat. 2016 Mar;228(3):474-86. doi: 10.1111/joa.12418. Epub 2015 Nov 27. J Anat. 2016. PMID: 26610370 Free PMC article.
-
R-spondins secreted by human pancreas-derived mesenchymal stromal cells support pancreatic organoid proliferation.Cell Mol Life Sci. 2025 Mar 20;82(1):125. doi: 10.1007/s00018-025-05658-0. Cell Mol Life Sci. 2025. PMID: 40111532 Free PMC article.
-
Clinical and Molecular Attributes and Evaluation of Pancreatic Cystic Neoplasm.Biochim Biophys Acta Rev Cancer. 2023 Jan;1878(1):188851. doi: 10.1016/j.bbcan.2022.188851. Epub 2022 Dec 16. Biochim Biophys Acta Rev Cancer. 2023. PMID: 36535512 Free PMC article. Review.
-
Single-cell resolution analysis of the human pancreatic ductal progenitor cell niche.Proc Natl Acad Sci U S A. 2020 May 19;117(20):10876-10887. doi: 10.1073/pnas.1918314117. Epub 2020 Apr 30. Proc Natl Acad Sci U S A. 2020. PMID: 32354994 Free PMC article.
References
-
- Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–670. - PubMed
-
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. - PubMed
-
- Grapin-Botton A. Ductal cells of the pancreas. Int J Biochem Cell Biol. 2005;37:504–510. - PubMed
-
- Bonner-Weir S, Sharma A. Pancreatic stem cells. J Pathol. 2002;197:519–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

