Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab
- PMID: 26100356
- PMCID: PMC4596761
- DOI: 10.1158/2326-6066.CIR-15-0102
Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab
Abstract
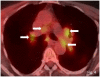
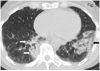
Ipilimumab is a promising novel immunotherapy agent and is associated with a variety of immune-related adverse events (irAE). The purpose of this study was to investigate the manifestations of irAEs on body imaging in patients with advanced melanoma treated with ipilimumab. One-hundred forty-seven patients with advanced melanoma (59 women, 88 men; median age, 64.5 years) treated with ipilimumab were studied. All patients had the baseline and at least one follow-up chest/abdomen/pelvis CT or PET/CT during therapy, which were reviewed by a consensus of two radiologists blinded to the clinical data. Findings indicative of individual types of irAEs were assessed, including thyroiditis, sarcoid-like lymphadenopathy, pneumonitis, hepatitis, pancreatitis, and colitis. Among the 147 patients, 46 (31%) had radiologically identified irAEs. The time interval from the initiation of therapy to the development of irAEs was less than 3 months in 76% (35 of 46) of the patients (range, 0.2-9.1 months). Clinical characteristics did not differ between patients with and without irAEs (P > 0.18). Among the individual types of irAEs, colitis was most common (n = 28; 19%), followed by sarcoid-like lymphadenopathy (n = 8; 5%) and pneumonitis (n = 8; 5%). Hepatitis (n = 3), thyroiditis (n = 2), and pancreatitis (n = 1) were less common. The resolution of irAEs was noted in 32 of 36 patients (89%) with further follow-up scans, with a median time of 2.3 months after the detection of irAE. In conclusion, irAEs were noted on body imaging in 31% of patients with melanoma treated with ipilimumab. Colitis was the most common, followed by sarcoid-like lymphadenopathy and pneumonitis. The results call for an increased awareness of irAEs, given the expanding role of cancer immunotherapy.
©2015 American Association for Cancer Research.
Figures



References
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. - PubMed
-
- Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–70. - PubMed
-
- Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Jr, Lombard LA, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–11. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

