Therapeutic antidepressant potential of a conjugated siRNA silencing the serotonin transporter after intranasal administration
- PMID: 26100539
- PMCID: PMC4759205
- DOI: 10.1038/mp.2015.80
Therapeutic antidepressant potential of a conjugated siRNA silencing the serotonin transporter after intranasal administration
Abstract
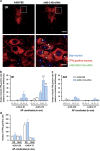
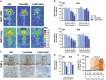
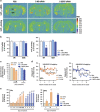
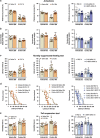
Major depression brings about a heavy socio-economic burden worldwide due to its high prevalence and the low efficacy of antidepressant drugs, mostly inhibiting the serotonin transporter (SERT). As a result, ~80% of patients show recurrent or chronic depression, resulting in a poor quality of life and increased suicide risk. RNA interference (RNAi) strategies have been preliminarily used to evoke antidepressant-like responses in experimental animals. However, the main limitation for the medical use of RNAi is the extreme difficulty to deliver oligonucleotides to selected neurons/systems in the mammalian brain. Here we show that the intranasal administration of a sertraline-conjugated small interfering RNA (C-SERT-siRNA) silenced SERT expression/function and evoked fast antidepressant-like responses in mice. After crossing the permeable olfactory epithelium, the sertraline-conjugated-siRNA was internalized and transported to serotonin cell bodies by deep Rab-7-associated endomembrane vesicles. Seven-day C-SERT-siRNA evoked similar or more marked responses than 28-day fluoxetine treatment. Hence, C-SERT-siRNA (i) downregulated 5-HT1A-autoreceptors and facilitated forebrain serotonin neurotransmission, (ii) accelerated the proliferation of neuronal precursors and (iii) increased hippocampal complexity and plasticity. Further, short-term C-SERT-siRNA reversed depressive-like behaviors in corticosterone-treated mice. The present results show the feasibility of evoking antidepressant-like responses by selectively targeting neuronal populations with appropriate siRNA strategies, opening a way for further translational studies.
Conflict of interest statement
FA has received consulting and educational honoraria on antidepressant drugs from Lundbeck and he is PI of grants from Lundbeck. He is also a member of the advisory board of Neurolixis Inc. AB and FA are authors of the patent WO/2011/131693 for the siRNA and ASO (antisense oligonucleotides) molecules and the targeting approach related to this work. GA and AM are board members of nLife Therapeutics S.L. The rest of authors declare no competing financial interest.
Figures

References
-
- 1Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci 2001; 2: 343–351. - PubMed
-
- 2Belmaker RH, Agam G. Major depressive disorder. N Engl J Med 2008; 358: 55–68. - PubMed
-
- 4Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatry Res 2003; 37: 357–373. - PubMed
-
- 5Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol 1994; 196: 263–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

