Molecular Determinants for the Inactivation of the Retinoblastoma Tumor Suppressor by the Viral Cyclin-dependent Kinase UL97
- PMID: 26100623
- PMCID: PMC4528131
- DOI: 10.1074/jbc.M115.660043
Molecular Determinants for the Inactivation of the Retinoblastoma Tumor Suppressor by the Viral Cyclin-dependent Kinase UL97
Abstract
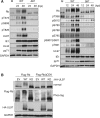
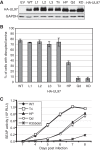
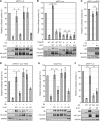
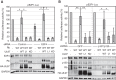
The retinoblastoma (Rb) tumor suppressor restricts cell cycle progression by repressing E2F-responsive transcription. Cellular cyclin-dependent kinase (CDK)-mediated Rb inactivation through phosphorylation disrupts Rb-E2F complexes, stimulating transcription. The human cytomegalovirus (HCMV) UL97 protein is a viral CDK (v-CDK) that phosphorylates Rb. Here we show that UL97 phosphorylates 11 of the 16 consensus CDK sites in Rb. A cleft within Rb accommodates peptides with the amino acid sequence LXCXE. UL97 contains three such motifs. We determined that the first LXCXE motif (L1) of UL97 and the Rb cleft enhance UL97-mediated Rb phosphorylation. A UL97 mutant with a non-functional L1 motif (UL97-L1m) displayed significantly reduced Rb phosphorylation at multiple sites. Curiously, however, it efficiently disrupted Rb-E2F complexes but failed to relieve Rb-mediated repression of E2F reporter constructs. The HCMV immediate early 1 protein cooperated with UL97-L1m to inactivate Rb in transfection assays, likely indicating that cells infected with a UL97-L1m mutant virus show no defects in growth or E2F-responsive gene expression because of redundant viral mechanisms to inactivate Rb. Our data suggest that UL97 possesses a mechanism to elicit E2F-dependent gene expression distinct from disruption of Rb-E2F complexes and dependent upon both the L1 motif of UL97 and the cleft region of Rb.
Keywords: cancer; cyclin-dependent kinase (CDK); cytomegalovirus; herpesvirus; oncogene; phosphorylation; retinoblastoma protein (pRb, RB); transcription.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Human cytomegalovirus-encoded viral cyclin-dependent kinase (v-CDK) UL97 phosphorylates and inactivates the retinoblastoma protein-related p107 and p130 proteins.J Biol Chem. 2017 Apr 21;292(16):6583-6599. doi: 10.1074/jbc.M116.773150. Epub 2017 Mar 13. J Biol Chem. 2017. PMID: 28289097 Free PMC article.
-
Human Cytomegalovirus Can Procure Deoxyribonucleotides for Viral DNA Replication in the Absence of Retinoblastoma Protein Phosphorylation.J Virol. 2016 Sep 12;90(19):8634-43. doi: 10.1128/JVI.00731-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27440891 Free PMC article.
-
Phosphorylation of transcriptional regulators in the retinoblastoma protein pathway by UL97, the viral cyclin-dependent kinase encoded by human cytomegalovirus.Virology. 2017 Dec;512:95-103. doi: 10.1016/j.virol.2017.09.009. Virology. 2017. PMID: 28946006 Free PMC article.
-
Regulation of the retinoblastoma-E2F pathway by the ubiquitin-proteasome system.Biochim Biophys Acta. 2015 Oct;1849(10):1289-97. doi: 10.1016/j.bbagrm.2015.08.008. Epub 2015 Aug 28. Biochim Biophys Acta. 2015. PMID: 26319102 Review.
-
Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir.Rev Med Virol. 2009 Jul;19(4):215-29. doi: 10.1002/rmv.615. Rev Med Virol. 2009. PMID: 19434630 Free PMC article. Review.
Cited by
-
Emerging Mechanisms of G1/S Cell Cycle Control by Human and Mouse Cytomegaloviruses.mBio. 2021 Dec 21;12(6):e0293421. doi: 10.1128/mBio.02934-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903047 Free PMC article. Review.
-
Virus-host protein interactions as footprints of human cytomegalovirus replication.Curr Opin Virol. 2022 Feb;52:135-147. doi: 10.1016/j.coviro.2021.11.016. Epub 2021 Dec 16. Curr Opin Virol. 2022. PMID: 34923282 Free PMC article. Review.
-
Deficiencies in Cellular Processes Modulated by the Retinoblastoma Protein Do Not Account for Reduced Human Cytomegalovirus Replication in Its Absence.J Virol. 2015 Dec;89(23):11965-74. doi: 10.1128/JVI.01718-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378180 Free PMC article.
-
Porphyromonas gingivalis and Human Cytomegalovirus Co-Infection: A Potential Link Between Periodontal Disease and Oral Cancer Development.Cancers (Basel). 2025 Apr 30;17(9):1525. doi: 10.3390/cancers17091525. Cancers (Basel). 2025. PMID: 40361452 Free PMC article. Review.
-
Human Cytomegalovirus Primary Infection and Reactivation: Insights From Virion-Carried Molecules.Front Microbiol. 2020 Jul 14;11:1511. doi: 10.3389/fmicb.2020.01511. eCollection 2020. Front Microbiol. 2020. PMID: 32765441 Free PMC article. Review.
References
-
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. (1986) A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 323, 643–646 - PubMed
-
- Weinberg R. A. (1995) The retinoblastoma protein and cell cycle control. Cell 81, 323–330 - PubMed
-
- Di Fiore R., D'Anneo A., Tesoriere G., Vento R. (2013) RB1 in cancer: different mechanisms of RB1 inactivation and alterations of pRb pathway in tumorigenesis. J. Cell. Physiol. 228, 1676–1687 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

