Swimming muscles power suction feeding in largemouth bass
- PMID: 26100863
- PMCID: PMC4507191
- DOI: 10.1073/pnas.1508055112
Swimming muscles power suction feeding in largemouth bass
Abstract
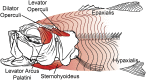
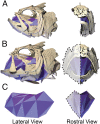
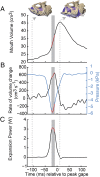
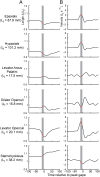
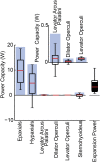
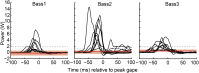
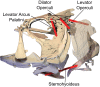
Most aquatic vertebrates use suction to capture food, relying on rapid expansion of the mouth cavity to accelerate water and food into the mouth. In ray-finned fishes, mouth expansion is both fast and forceful, and therefore requires considerable power. However, the cranial muscles of these fishes are relatively small and may not be able to produce enough power for suction expansion. The axial swimming muscles of these fishes also attach to the feeding apparatus and have the potential to generate mouth expansion. Because of their large size, these axial muscles could contribute substantial power to suction feeding. To determine whether suction feeding is powered primarily by axial muscles, we measured the power required for suction expansion in largemouth bass and compared it to the power capacities of the axial and cranial muscles. Using X-ray reconstruction of moving morphology (XROMM), we generated 3D animations of the mouth skeleton and created a dynamic digital endocast to measure the rate of mouth volume expansion. This time-resolved expansion rate was combined with intraoral pressure recordings to calculate the instantaneous power required for suction feeding. Peak expansion powers for all but the weakest strikes far exceeded the maximum power capacity of the cranial muscles. The axial muscles did not merely contribute but were the primary source of suction expansion power and generated up to 95% of peak expansion power. The recruitment of axial muscle power may have been crucial for the evolution of high-power suction feeding in ray-finned fishes.
Keywords: XROMM; epaxial; hypaxial; muscle power; volume.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
How fish power suction feeding.Proc Natl Acad Sci U S A. 2015 Jul 14;112(28):8525-6. doi: 10.1073/pnas.1510522112. Epub 2015 Jul 6. Proc Natl Acad Sci U S A. 2015. PMID: 26150493 Free PMC article. No abstract available.
Similar articles
-
Role of axial muscles in powering mouth expansion during suction feeding in largemouth bass (Micropterus salmoides).J Exp Biol. 2014 Apr 15;217(Pt 8):1333-45. doi: 10.1242/jeb.095810. Epub 2013 Dec 20. J Exp Biol. 2014. PMID: 24363416
-
Fishes can use axial muscles as anchors or motors for powerful suction feeding.J Exp Biol. 2020 Sep 18;223(Pt 18):jeb225649. doi: 10.1242/jeb.225649. J Exp Biol. 2020. PMID: 32948649 Free PMC article.
-
Reevaluating Musculoskeletal Linkages in Suction-Feeding Fishes with X-Ray Reconstruction of Moving Morphology (XROMM).Integr Comp Biol. 2015 Jul;55(1):36-47. doi: 10.1093/icb/icv034. Epub 2015 May 13. Integr Comp Biol. 2015. PMID: 25972567
-
Morphology, Kinematics, and Dynamics: The Mechanics of Suction Feeding in Fishes.Integr Comp Biol. 2015 Jul;55(1):21-35. doi: 10.1093/icb/icv032. Epub 2015 May 16. Integr Comp Biol. 2015. PMID: 25980568 Review.
-
Origins, Innovations, and Diversification of Suction Feeding in Vertebrates.Integr Comp Biol. 2015 Jul;55(1):134-45. doi: 10.1093/icb/icv026. Epub 2015 Apr 27. Integr Comp Biol. 2015. PMID: 25920508 Review.
Cited by
-
Functional groups in piscivorous fishes.Ecol Evol. 2021 Sep 2;11(18):12765-12778. doi: 10.1002/ece3.8020. eCollection 2021 Sep. Ecol Evol. 2021. PMID: 34594537 Free PMC article.
-
Channel catfish use higher coordination to capture prey than to swallow.Proc Biol Sci. 2019 Apr 24;286(1901):20190507. doi: 10.1098/rspb.2019.0507. Proc Biol Sci. 2019. PMID: 30991933 Free PMC article.
-
Axial muscle-fibre orientations in larval zebrafish.J Anat. 2025 Apr;246(4):517-533. doi: 10.1111/joa.14161. Epub 2024 Nov 18. J Anat. 2025. PMID: 39556060 Free PMC article.
-
Covariation of brain and skull shapes as a model to understand the role of crosstalk in development and evolution.Evol Dev. 2023 Jan;25(1):85-102. doi: 10.1111/ede.12421. Epub 2022 Nov 14. Evol Dev. 2023. PMID: 36377237 Free PMC article.
-
Suction feeding of West African lungfish (Protopterus annectens): An XROMM analysis of jaw mechanics, cranial kinesis, and hyoid mobility.Biol Open. 2022 Sep 15;11(9):bio059447. doi: 10.1242/bio.059447. Epub 2022 Sep 12. Biol Open. 2022. PMID: 36066131 Free PMC article.
References
-
- Askew GN, Marsh RL. The mechanical power output of the pectoralis muscle of blue-breasted quail (Coturnix chinensis): The in vivo length cycle and its implications for muscle performance. J Exp Biol. 2001;204(Pt 21):3587–3600. - PubMed
-
- Aerts P. Vertical jumping in Galago senegalensis: The quest for an obligate mechanical power amplifier. Philos Trans R Soc Lond B Biol Sci. 1998;353(1375):1607–1620.
-
- Swoap SJ, Johnson TP, Josephson RK, Bennett AF. Temperature, muscle power output and limitations on burst locomotor performance of Dipsosaurus dorsalis. J Exp Biol. 1993;174(1):185–197.
-
- Herrel A, McBrayer LD, Larson PM. Functional basis for sexual differences in bite force in the lizard Anolis carolinensis. Biol J Linn Soc Lond. 2007;91(1):111–119.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

