Pathways to dewetting in hydrophobic confinement
- PMID: 26100866
- PMCID: PMC4500207
- DOI: 10.1073/pnas.1503302112
Pathways to dewetting in hydrophobic confinement
Abstract
Liquid water can become metastable with respect to its vapor in hydrophobic confinement. The resulting dewetting transitions are often impeded by large kinetic barriers. According to macroscopic theory, such barriers arise from the free energy required to nucleate a critical vapor tube that spans the region between two hydrophobic surfaces--tubes with smaller radii collapse, whereas larger ones grow to dry the entire confined region. Using extensive molecular simulations of water between two nanoscopic hydrophobic surfaces, in conjunction with advanced sampling techniques, here we show that for intersurface separations that thermodynamically favor dewetting, the barrier to dewetting does not correspond to the formation of a (classical) critical vapor tube. Instead, it corresponds to an abrupt transition from an isolated cavity adjacent to one of the confining surfaces to a gap-spanning vapor tube that is already larger than the critical vapor tube anticipated by macroscopic theory. Correspondingly, the barrier to dewetting is also smaller than the classical expectation. We show that the peculiar nature of water density fluctuations adjacent to extended hydrophobic surfaces--namely, the enhanced likelihood of observing low-density fluctuations relative to Gaussian statistics--facilitates this nonclassical behavior. By stabilizing isolated cavities relative to vapor tubes, enhanced water density fluctuations thus stabilize novel pathways, which circumvent the classical barriers and offer diminished resistance to dewetting. Our results thus suggest a key role for fluctuations in speeding up the kinetics of numerous phenomena ranging from Cassie-Wenzel transitions on superhydrophobic surfaces, to hydrophobically driven biomolecular folding and assembly.
Keywords: assembly; capillary evaporation; fluctuations; kinetic barriers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

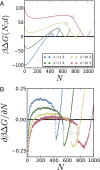
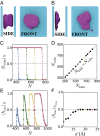
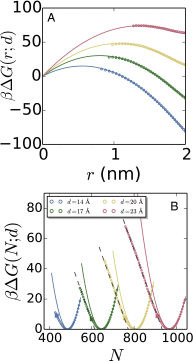
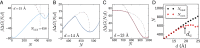
References
-
- Tanford C. The Hydrophobic Effect - Formation of Micelles and Biological Membranes. Wiley Interscience; New York: 1973.
-
- Kauzmann W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. - PubMed
-
- Stillinger FH. Structure in aqueous solutions of nonpolar solutes from the standpoint of scaled-particle theory. J Solution Chem. 1973;2:141–158.
-
- Israelachvili JN. Intermolecular and surface forces. revised third edition Academic, Waltham, MA; 2011.
-
- Chandler D. Interfaces and the driving force of hydrophobic assembly. Nature. 2005;437(7059):640–647. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

