Theta-gamma coordination between anterior cingulate and prefrontal cortex indexes correct attention shifts
- PMID: 26100868
- PMCID: PMC4500211
- DOI: 10.1073/pnas.1500438112
Theta-gamma coordination between anterior cingulate and prefrontal cortex indexes correct attention shifts
Abstract
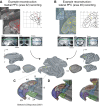
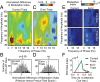
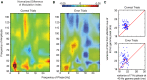
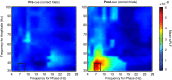
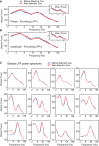
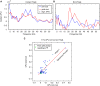
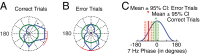
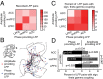
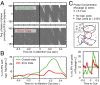
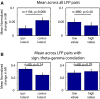
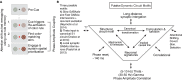
Anterior cingulate and lateral prefrontal cortex (ACC/PFC) are believed to coordinate activity to flexibly prioritize the processing of goal-relevant over irrelevant information. This between-area coordination may be realized by common low-frequency excitability changes synchronizing segregated high-frequency activations. We tested this coordination hypothesis by recording in macaque ACC/PFC during the covert utilization of attention cues. We found robust increases of 5-10 Hz (theta) to 35-55 Hz (gamma) phase-amplitude correlation between ACC and PFC during successful attention shifts but not before errors. Cortical sites providing theta phases (i) showed a prominent cue-induced phase reset, (ii) were more likely in ACC than PFC, and (iii) hosted neurons with burst firing events that synchronized to distant gamma activity. These findings suggest that interareal theta-gamma correlations could follow mechanistically from a cue-triggered reactivation of rule memory that synchronizes theta across ACC/PFC.
Keywords: anterior cingulate cortex; attention; gamma oscillation; prefrontal cortex; theta oscillation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Interareal Spike-Train Correlations of Anterior Cingulate and Dorsal Prefrontal Cortex during Attention Shifts.J Neurosci. 2015 Sep 23;35(38):13076-89. doi: 10.1523/JNEUROSCI.1262-15.2015. J Neurosci. 2015. PMID: 26400938 Free PMC article.
-
Burst firing synchronizes prefrontal and anterior cingulate cortex during attentional control.Curr Biol. 2014 Nov 17;24(22):2613-21. doi: 10.1016/j.cub.2014.09.046. Epub 2014 Oct 9. Curr Biol. 2014. PMID: 25308081
-
Cell-Type Specific Burst Firing Interacts with Theta and Beta Activity in Prefrontal Cortex During Attention States.Cereb Cortex. 2018 Dec 1;28(12):4348-4364. doi: 10.1093/cercor/bhx287. Cereb Cortex. 2018. PMID: 29136106 Free PMC article.
-
Comparing the prefrontal cortex of rats and primates: insights from electrophysiology.Neurotox Res. 2008 Oct;14(2-3):249-62. doi: 10.1007/BF03033814. Neurotox Res. 2008. PMID: 19073430 Review.
-
Reward-dependent learning in neuronal networks for planning and decision making.Prog Brain Res. 2000;126:217-29. doi: 10.1016/S0079-6123(00)26016-0. Prog Brain Res. 2000. PMID: 11105649 Review.
Cited by
-
Spike Timing in the Attention Network Predicts Behavioral Outcome Prior to Target Selection.Neuron. 2021 Jan 6;109(1):177-188.e4. doi: 10.1016/j.neuron.2020.09.039. Epub 2020 Oct 23. Neuron. 2021. PMID: 33098762 Free PMC article.
-
Theta oscillations shift towards optimal frequency for cognitive control.Nat Hum Behav. 2022 Jul;6(7):1000-1013. doi: 10.1038/s41562-022-01335-5. Epub 2022 Apr 21. Nat Hum Behav. 2022. PMID: 35449299
-
An Integrative Model of Effortful Control.Front Syst Neurosci. 2019 Dec 20;13:79. doi: 10.3389/fnsys.2019.00079. eCollection 2019. Front Syst Neurosci. 2019. PMID: 31920573 Free PMC article.
-
Synchronization between frontal eye field and area V4 during free-gaze visual search.Zool Res. 2019 Sep 18;40(5):394-403. doi: 10.24272/j.issn.2095-8137.2019.055. Zool Res. 2019. PMID: 31393096 Free PMC article.
-
Solving the Credit Assignment Problem With the Prefrontal Cortex.Front Neurosci. 2018 Mar 27;12:182. doi: 10.3389/fnins.2018.00182. eCollection 2018. Front Neurosci. 2018. PMID: 29636659 Free PMC article. Review.
References
-
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70(6):1054–1069. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

