N-linked glycosylation of protease-activated receptor-1 at extracellular loop 2 regulates G-protein signaling bias
- PMID: 26100877
- PMCID: PMC4500229
- DOI: 10.1073/pnas.1508838112
N-linked glycosylation of protease-activated receptor-1 at extracellular loop 2 regulates G-protein signaling bias
Abstract
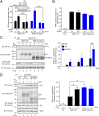
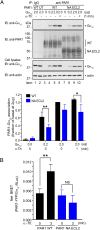
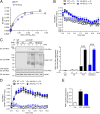
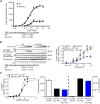
Protease-activated receptor-1 (PAR1) is a G-protein-coupled receptor (GPCR) for the coagulant protease thrombin. Similar to other GPCRs, PAR1 is promiscuous and couples to multiple heterotrimeric G-protein subtypes in the same cell and promotes diverse cellular responses. The molecular mechanism by which activation of a given GPCR with the same ligand permits coupling to multiple G-protein subtypes is unclear. Here, we report that N-linked glycosylation of PAR1 at extracellular loop 2 (ECL2) controls G12/13 versus Gq coupling specificity in response to thrombin stimulation. A PAR1 mutant deficient in glycosylation at ECL2 was more effective at stimulating Gq-mediated phosphoinositide signaling compared with glycosylated wildtype receptor. In contrast, wildtype PAR1 displayed a greater efficacy at G12/13-dependent RhoA activation compared with mutant receptor lacking glycosylation at ECL2. Endogenous PAR1 rendered deficient in glycosylation using tunicamycin, a glycoprotein synthesis inhibitor, also exhibited increased PI signaling and diminished RhoA activation opposite to native receptor. Remarkably, PAR1 wildtype and glycosylation-deficient mutant were equally effective at coupling to Gi and β-arrestin-1. Consistent with preferential G12/13 coupling, thrombin-stimulated PAR1 wildtype strongly induced RhoA-mediated stress fiber formation compared with mutant receptor. In striking contrast, glycosylation-deficient PAR1 was more effective at increasing cellular proliferation, associated with Gq signaling, than wildtype receptor. These studies suggest that N-linked glycosylation at ECL2 contributes to the stabilization of an active PAR1 state that preferentially couples to G12/13 versus Gq and defines a previously unidentified function for N-linked glycosylation of GPCRs in regulating G-protein signaling bias.
Keywords: GPCR; RhoA; arrestin; endothelial; thrombin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11.J Biol Chem. 2003 Aug 1;278(31):28743-9. doi: 10.1074/jbc.M304570200. Epub 2003 May 27. J Biol Chem. 2003. PMID: 12771155
-
N-linked glycosylation of protease-activated receptor-1 second extracellular loop: a critical determinant for ligand-induced receptor activation and internalization.J Biol Chem. 2010 Jun 11;285(24):18781-93. doi: 10.1074/jbc.M110.111088. Epub 2010 Apr 5. J Biol Chem. 2010. PMID: 20368337 Free PMC article.
-
Protease-activated receptor 1 (PAR1) coupling to G(q/11) but not to G(i/o) or G(12/13) is mediated by discrete amino acids within the receptor second intracellular loop.Cell Signal. 2012 Jun;24(6):1351-60. doi: 10.1016/j.cellsig.2012.01.011. Epub 2012 Jan 28. Cell Signal. 2012. PMID: 22306780 Free PMC article.
-
Molecular basis of protease-activated receptor 1 signaling diversity.J Thromb Haemost. 2020 Jan;18(1):6-16. doi: 10.1111/jth.14643. Epub 2019 Oct 17. J Thromb Haemost. 2020. PMID: 31549766 Review.
-
From Multiple PAR1 Receptor/Protein Interactions to their Multiple Therapeutic Implications.Curr Top Med Chem. 2015;15(20):2080-114. doi: 10.2174/1568026615666150519103911. Curr Top Med Chem. 2015. PMID: 25986685 Review.
Cited by
-
International Union of Basic and Clinical Pharmacology. CVII. Structure and Pharmacology of the Apelin Receptor with a Recommendation that Elabela/Toddler Is a Second Endogenous Peptide Ligand.Pharmacol Rev. 2019 Oct;71(4):467-502. doi: 10.1124/pr.119.017533. Pharmacol Rev. 2019. PMID: 31492821 Free PMC article. Review.
-
Effects of two different variants in the MAGT1 gene on B cell subsets, platelet function, and cell glycome composition.Front Immunol. 2025 Mar 18;16:1547808. doi: 10.3389/fimmu.2025.1547808. eCollection 2025. Front Immunol. 2025. PMID: 40170846 Free PMC article.
-
High endogenous activated protein C levels attenuates bleomycin-induced pulmonary fibrosis.J Cell Mol Med. 2016 Nov;20(11):2029-2035. doi: 10.1111/jcmm.12891. Epub 2016 Jun 14. J Cell Mol Med. 2016. PMID: 27295971 Free PMC article.
-
Calcitonin Receptor N-Glycosylation Enhances Peptide Hormone Affinity by Controlling Receptor Dynamics.J Mol Biol. 2020 Mar 27;432(7):1996-2014. doi: 10.1016/j.jmb.2020.01.028. Epub 2020 Feb 6. J Mol Biol. 2020. PMID: 32035902 Free PMC article.
-
N-Glycosylation of Asparagine 130 in the Extracellular Domain of the Human Calcitonin Receptor Significantly Increases Peptide Hormone Affinity.Biochemistry. 2017 Jul 5;56(26):3380-3393. doi: 10.1021/acs.biochem.7b00256. Epub 2017 Jun 26. Biochemistry. 2017. PMID: 28614667 Free PMC article.
References
-
- Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3(8):1800–1814. - PubMed
-
- Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Targeting proteinase-activated receptors: Therapeutic potential and challenges. Nat Rev Drug Discov. 2012;11(1):69–86. - PubMed
-
- Arora P, Ricks TK, Trejo J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J Cell Sci. 2007;120(Pt 6):921–928. - PubMed
-
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64(6):1057–1068. - PubMed
-
- Vu TK, Wheaton VI, Hung DT, Charo I, Coughlin SR. Domains specifying thrombin-receptor interaction. Nature. 1991;353(6345):674–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

