Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer
- PMID: 26100888
- PMCID: PMC4500251
- DOI: 10.1073/pnas.1507704112
Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer
Abstract
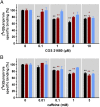
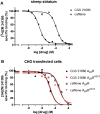
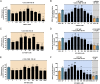
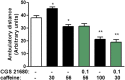
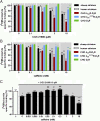
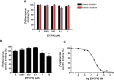
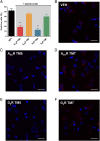
Adenosine A2A receptor (A2AR)-dopamine D2 receptor (D2R) heteromers are key modulators of striatal neuronal function. It has been suggested that the psychostimulant effects of caffeine depend on its ability to block an allosteric modulation within the A2AR-D2R heteromer, by which adenosine decreases the affinity and intrinsic efficacy of dopamine at the D2R. We describe novel unsuspected allosteric mechanisms within the heteromer by which not only A2AR agonists, but also A2AR antagonists, decrease the affinity and intrinsic efficacy of D2R agonists and the affinity of D2R antagonists. Strikingly, these allosteric modulations disappear on agonist and antagonist coadministration. This can be explained by a model that considers A2AR-D2R heteromers as heterotetramers, constituted by A2AR and D2R homodimers, as demonstrated by experiments with bioluminescence resonance energy transfer and bimolecular fluorescence and bioluminescence complementation. As predicted by the model, high concentrations of A2AR antagonists behaved as A2AR agonists and decreased D2R function in the brain.
Keywords: GPCR heteromers; adenosine A2A receptor; caffeine; dopamine D2 receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Allosteric mechanisms within the adenosine A2A-dopamine D2 receptor heterotetramer.Neuropharmacology. 2016 May;104:154-60. doi: 10.1016/j.neuropharm.2015.05.028. Epub 2015 Jun 4. Neuropharmacology. 2016. PMID: 26051403 Free PMC article. Review.
-
Allosteric Interactions between Adenosine A2A and Dopamine D2 Receptors in Heteromeric Complexes: Biochemical and Pharmacological Characteristics, and Opportunities for PET Imaging.Int J Mol Sci. 2021 Feb 9;22(4):1719. doi: 10.3390/ijms22041719. Int J Mol Sci. 2021. PMID: 33572077 Free PMC article. Review.
-
Evidence for the heterotetrameric structure of the adenosine A2A-dopamine D2 receptor complex.Biochem Soc Trans. 2016 Apr 15;44(2):595-600. doi: 10.1042/BST20150276. Biochem Soc Trans. 2016. PMID: 27068975 Review.
-
Modulation of A₂a receptor antagonist on D₂ receptor internalization and ERK phosphorylation.Acta Pharmacol Sin. 2013 Oct;34(10):1292-300. doi: 10.1038/aps.2013.87. Epub 2013 Aug 12. Acta Pharmacol Sin. 2013. PMID: 23933651 Free PMC article.
-
Acute cocaine treatment enhances the antagonistic allosteric adenosine A2A-dopamine D2 receptor-receptor interactions in rat dorsal striatum without increasing significantly extracellular dopamine levels.Pharmacol Rep. 2020 Apr;72(2):332-339. doi: 10.1007/s43440-020-00069-3. Epub 2020 Mar 2. Pharmacol Rep. 2020. PMID: 32124388
Cited by
-
Signaling models for dopamine-dependent temporal contiguity in striatal synaptic plasticity.PLoS Comput Biol. 2020 Jul 23;16(7):e1008078. doi: 10.1371/journal.pcbi.1008078. eCollection 2020 Jul. PLoS Comput Biol. 2020. PMID: 32701987 Free PMC article.
-
Overcoming the Challenges of Detecting GPCR Oligomerization in the Brain.Curr Neuropharmacol. 2022;20(6):1035-1045. doi: 10.2174/1570159X19666211104145727. Curr Neuropharmacol. 2022. PMID: 34736381 Free PMC article.
-
Hormones and Neuropeptide Receptor Heteromers in the Ventral Tegmental Area. Targets for the Treatment of Loss of Control of Food Intake and Substance Use Disorders.Curr Treat Options Psychiatry. 2017;4(2):167-183. doi: 10.1007/s40501-017-0109-x. Epub 2017 Apr 22. Curr Treat Options Psychiatry. 2017. PMID: 28580231 Free PMC article. Review.
-
The First Negative Allosteric Modulator for Dopamine D2 and D3 Receptors, SB269652 May Lead to a New Generation of Antipsychotic Drugs.Mol Pharmacol. 2017 Jun;91(6):586-594. doi: 10.1124/mol.116.107607. Epub 2017 Mar 6. Mol Pharmacol. 2017. PMID: 28265019 Free PMC article. Review.
-
Apelin receptor homodimer-oligomers revealed by single-molecule imaging and novel G protein-dependent signaling.Sci Rep. 2017 Jan 16;7:40335. doi: 10.1038/srep40335. Sci Rep. 2017. PMID: 28091541 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

