Antibiotic efficacy is linked to bacterial cellular respiration
- PMID: 26100898
- PMCID: PMC4500273
- DOI: 10.1073/pnas.1509743112
Antibiotic efficacy is linked to bacterial cellular respiration
Abstract
Bacteriostatic and bactericidal antibiotic treatments result in two fundamentally different phenotypic outcomes--the inhibition of bacterial growth or, alternatively, cell death. Most antibiotics inhibit processes that are major consumers of cellular energy output, suggesting that antibiotic treatment may have important downstream consequences on bacterial metabolism. We hypothesized that the specific metabolic effects of bacteriostatic and bactericidal antibiotics contribute to their overall efficacy. We leveraged the opposing phenotypes of bacteriostatic and bactericidal drugs in combination to investigate their activity. Growth inhibition from bacteriostatic antibiotics was associated with suppressed cellular respiration whereas cell death from most bactericidal antibiotics was associated with accelerated respiration. In combination, suppression of cellular respiration by the bacteriostatic antibiotic was the dominant effect, blocking bactericidal killing. Global metabolic profiling of bacteriostatic antibiotic treatment revealed that accumulation of metabolites involved in specific drug target activity was linked to the buildup of energy metabolites that feed the electron transport chain. Inhibition of cellular respiration by knockout of the cytochrome oxidases was sufficient to attenuate bactericidal lethality whereas acceleration of basal respiration by genetically uncoupling ATP synthesis from electron transport resulted in potentiation of the killing effect of bactericidal antibiotics. This work identifies a link between antibiotic-induced cellular respiration and bactericidal lethality and demonstrates that bactericidal activity can be arrested by attenuated respiration and potentiated by accelerated respiration. Our data collectively show that antibiotics perturb the metabolic state of bacteria and that the metabolic state of bacteria impacts antibiotic efficacy.
Keywords: E. coli; S. aureus; antibiotics; cellular respiration; metabolomics.
Conflict of interest statement
Conflict of interest statement: J.J.C. is a scientific cofounder and Scientific Advisory Board chair of EnBiotix, Inc., a start-up focused on antibiotic development.
Figures

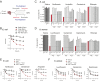
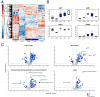
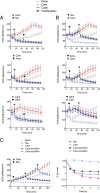
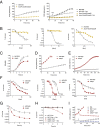
Comment in
-
Bactericidal antibiotics induce programmed metabolic toxicity.Microb Cell. 2016 Mar 9;3(4):178-180. doi: 10.15698/mic2016.04.493. Microb Cell. 2016. PMID: 28357350 Free PMC article.
References
-
- Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38(6):864–870. - PubMed
-
- Finberg RW, et al. The importance of bactericidal drugs: Future directions in infectious disease. Clin Infect Dis. 2004;39(9):1314–1320. - PubMed
-
- Chowdhury MH, Tunkel AR. Antibacterial agents in infections of the central nervous system. Infect Dis Clin North Am. 2000;14(2):391–408. - PubMed
-
- Archer G, Fekety FR., Jr Experimental endocarditis due to Pseudomonas aeruginosa. II. Therapy with carbenicillin and gentamicin. J Infect Dis. 1977;136(3):327–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

