BEWARE: Body awareness training in the treatment of wearing-off related anxiety in patients with Parkinson's disease: study protocol for a randomized controlled trial
- PMID: 26101038
- PMCID: PMC4489048
- DOI: 10.1186/s13063-015-0804-0
BEWARE: Body awareness training in the treatment of wearing-off related anxiety in patients with Parkinson's disease: study protocol for a randomized controlled trial
Abstract
Background: The wearing-off phenomenon in patients with Parkinson's disease (PD) is a complication of prolonged levodopa usage. During this phenomenon, motor symptoms such as rigidity and freezing re-emerge. This is often accompanied by non-motor symptoms, including anxiety, the so-called wearing-off related anxiety (WRA). Current treatment options are limited and typically focus on either the physical or mental aspects of wearing-off. An integrated approach seems warranted in order to optimally address the complex reciprocal interactions between these aspects. Also, because wearing-off is eventually inescapable, treatment needs to focus on coping, acceptance, and self-efficacy. We therefore developed an integrated body awareness intervention, combining principles from physical therapy with acceptance and commitment therapy to teach patients to deal with WRA. This study will investigate whether this new intervention, named BEWARE, is more effective than treatment as usual in increasing self-efficacy.
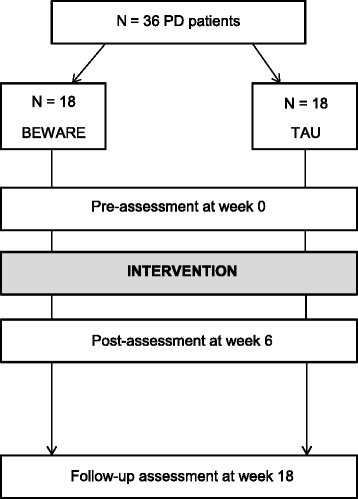
Methods/design: This is a single-blinded randomized controlled trial in 36 PD patients who experience WRA. Subjects will be recruited from the outpatient clinic for movement disorders of the VU University Medical Center. After providing written informed consent, patients will be randomly assigned to an experimental (BEWARE) or treatment-as-usual (physical therapy) group. Clinical assessments will be performed prior to the intervention, directly after the 6-week intervention period, and at 3-month naturalistic follow-up by a blinded investigator not involved in the study. The primary outcome measure is self-efficacy, and secondary outcomes focus on mobility, daily functioning, anxiety, and quality of life.
Discussion: Because wearing-off is an inevitable consequence of levodopa therapy and current treatment options are insufficient, a multidisciplinary intervention that addresses both physical and mental aspects of wearing-off in PD may foster additional benefits for treating WRA in PD patients over mono-disciplinary care alone.
Trial registration: ClinicalTrials.gov identifier: NCT02054845. Date of registration: 30 January 2014.
Figures
Similar articles
-
Body awareness training in the treatment of wearing-off related anxiety in patients with Parkinson's disease: Results from a pilot randomized controlled trial.J Psychosom Res. 2017 Dec;103:1-8. doi: 10.1016/j.jpsychores.2017.09.008. Epub 2017 Sep 21. J Psychosom Res. 2017. PMID: 29167034 Clinical Trial.
-
Evaluating the impact of adjunctive istradefylline on the cumulative dose of levodopa-containing medications in Parkinson's disease: study protocol for the ISTRA ADJUST PD randomized, controlled study.BMC Neurol. 2022 Mar 3;22(1):71. doi: 10.1186/s12883-022-02600-w. BMC Neurol. 2022. PMID: 35241003 Free PMC article.
-
Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson's disease.Mov Disord. 2013 Jul;28(8):1064-71. doi: 10.1002/mds.25364. Epub 2013 Apr 29. Mov Disord. 2013. PMID: 23630119 Clinical Trial.
-
Levodopa-related wearing-off in Parkinson's disease: identification and management.Curr Med Res Opin. 2009 Apr;25(4):841-9. doi: 10.1185/03007990902779319. Curr Med Res Opin. 2009. PMID: 19228103 Review.
-
Role of the pharmacist in the effective management of wearing-off in Parkinson's disease.Ann Pharmacother. 2007 Nov;41(11):1842-9. doi: 10.1345/aph.1K348. Epub 2007 Sep 18. Ann Pharmacother. 2007. PMID: 17878397 Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical