Individual differences in cortical connections of somatosensory cortex are associated with parental rearing style in prairie voles (Microtus ochrogaster)
- PMID: 26101098
- PMCID: PMC4689663
- DOI: 10.1002/cne.23837
Individual differences in cortical connections of somatosensory cortex are associated with parental rearing style in prairie voles (Microtus ochrogaster)
Abstract
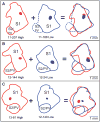
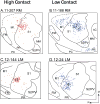
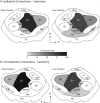
Early-life sensory experiences have a profound effect on brain organization, connectivity, and subsequent behavior. In most mammals, the earliest sensory inputs are delivered to the developing brain through tactile contact with the parents, especially the mother. Prairie voles (Microtus ochrogaster) are monogamous and, like humans, are biparental. Within the normal prairie vole population, both the type and the amount of interactions, particularly tactile contact, that parents have with their offspring vary. The question is whether these early and pervasive differences in tactile stimulation and social experience between parent and offspring are manifest in differences in cortical organization and connectivity. To address this question, we examined the cortical and callosal connections of the primary somatosensory area (S1) in high-contact (HC) and low-contact (LC) offspring using neuroanatomical tracing techniques. Injection sites within S1 were matched so that direct comparisons between these two groups could be made. We observed several important differences between these groups. The first was that HC offspring had a greater density of intrinsic connections within S1 compared with LC offspring. Additionally, HC offspring had a more restricted pattern of ipsilateral connections, whereas LC offspring had dense connections with areas of parietal and frontal cortex that were more widespread. Finally, LC offspring had a broader distribution of callosal connections than HC offspring and a significantly higher percentage of labeled callosal neurons. This study is the first to examine individual differences in cortical connections and suggests that individual differences in cortical connections may be related to natural differences in parental rearing styles associated with tactile contact.
Keywords: S1; development; epigenetics; individual differences; primary somatosensory cortex.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Figures







Similar articles
-
Early postnatal gene expression in the developing neocortex of prairie voles (Microtus ochrogaster) is related to parental rearing style.J Comp Neurol. 2020 Dec 1;528(17):3008-3022. doi: 10.1002/cne.24856. Epub 2020 Jan 24. J Comp Neurol. 2020. PMID: 31930725 Free PMC article.
-
Organization of the Perioral Representation of the Primary Somatosensory Cortex in Prairie Voles (<italic>Microtus ochrogaster</italic>).Brain Behav Evol. 2025;100(3):139-155. doi: 10.1159/000543248. Epub 2025 Jan 10. Brain Behav Evol. 2025. PMID: 39799933 Free PMC article.
-
Connections of auditory and visual cortex in the prairie vole (Microtus ochrogaster): evidence for multisensory processing in primary sensory areas.Cereb Cortex. 2010 Jan;20(1):89-108. doi: 10.1093/cercor/bhp082. Cereb Cortex. 2010. PMID: 19395525 Free PMC article.
-
Exploration in a dispersal task: Effects of early experience and correlation with other behaviors in prairie voles (Microtus ochrogaster).Behav Processes. 2016 Nov;132:66-75. doi: 10.1016/j.beproc.2016.10.002. Epub 2016 Oct 5. Behav Processes. 2016. PMID: 27720755 Free PMC article.
-
Neurochemical regulation of pair bonding in male prairie voles.Physiol Behav. 2004 Nov 15;83(2):319-28. doi: 10.1016/j.physbeh.2004.08.024. Physiol Behav. 2004. PMID: 15488548 Review.
Cited by
-
Hypergravity within a critical period impacts on the maturation of somatosensory cortical maps and their potential for use-dependent plasticity in the adult.J Neurophysiol. 2016 Jun 1;115(6):2740-60. doi: 10.1152/jn.00900.2015. Epub 2016 Feb 17. J Neurophysiol. 2016. PMID: 26888103 Free PMC article.
-
Localization of oxytocin receptors in the prairie vole (Microtus ochrogaster) neocortex.Neuroscience. 2017 Apr 21;348:201-211. doi: 10.1016/j.neuroscience.2017.02.017. Epub 2017 Feb 15. Neuroscience. 2017. PMID: 28214580 Free PMC article.
-
The developmental support hypothesis: adaptive plasticity in neural development in response to cues of social support.Philos Trans R Soc Lond B Biol Sci. 2020 Jul 20;375(1803):20190491. doi: 10.1098/rstb.2019.0491. Epub 2020 Jun 1. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32475336 Free PMC article. Review.
-
Intergenerational transmission of sociality: the role of parents in shaping social behavior in monogamous and non-monogamous species.J Exp Biol. 2017 Jan 1;220(Pt 1):114-123. doi: 10.1242/jeb.142182. J Exp Biol. 2017. PMID: 28057834 Free PMC article. Review.
-
Childhood Adversity and Affective Touch Perception: A Comparison of United Kingdom Care Leavers and Non-care Leavers.Front Psychol. 2020 Nov 10;11:557171. doi: 10.3389/fpsyg.2020.557171. eCollection 2020. Front Psychol. 2020. PMID: 33240148 Free PMC article.
References
-
- Adams DL, Horton JC. Capricious expression of cortical columns in the primate brain. Nat Neurosci. 2003;6(2):113–114. - PubMed
-
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44(3):178–184. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

