Evolution of posterior parietal cortex and parietal-frontal networks for specific actions in primates
- PMID: 26101180
- PMCID: PMC4689678
- DOI: 10.1002/cne.23838
Evolution of posterior parietal cortex and parietal-frontal networks for specific actions in primates
Abstract
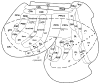
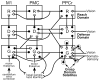
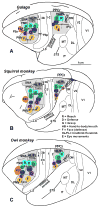
Posterior parietal cortex (PPC) is an extensive region of the human brain that develops relatively late and is proportionally large compared with that of monkeys and prosimian primates. Our ongoing comparative studies have led to several conclusions about the evolution of this posterior parietal region. In early placental mammals, PPC likely was a small multisensory region much like PPC of extant rodents and tree shrews. In early primates, PPC likely resembled that of prosimian galagos, in which caudal PPC (PPCc) is visual and rostral PPC (PPCr) has eight or more multisensory domains where electrical stimulation evokes different complex motor behaviors, including reaching, hand-to-mouth, looking, protecting the face or body, and grasping. These evoked behaviors depend on connections with functionally matched domains in premotor cortex (PMC) and motor cortex (M1). Domains in each region compete with each other, and a serial arrangement of domains allows different factors to influence motor outcomes successively. Similar arrangements of domains have been retained in New and Old World monkeys, and humans appear to have at least some of these domains. The great expansion and prolonged development of PPC in humans suggest the addition of functionally distinct territories. We propose that, across primates, PMC and M1 domains are second and third levels in a number of parallel, interacting networks for mediating and selecting one type of action over others.
Keywords: cortical connections; motor behavior; motor cortex; prosimian primates; visual cortex.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Cortical connections of the functional domain for climbing or running in posterior parietal cortex of galagos.J Comp Neurol. 2021 Jul 1;529(10):2789-2812. doi: 10.1002/cne.25123. Epub 2021 Mar 4. J Comp Neurol. 2021. PMID: 33550608 Free PMC article.
-
Cortical networks for ethologically relevant behaviors in primates.Am J Primatol. 2013 May;75(5):407-14. doi: 10.1002/ajp.22065. Epub 2012 Aug 3. Am J Primatol. 2013. PMID: 22865408 Free PMC article. Review.
-
Cortical Connections of the Caudal Portion of Posterior Parietal Cortex in Prosimian Galagos.Cereb Cortex. 2016 Jun;26(6):2753-77. doi: 10.1093/cercor/bhv132. Epub 2015 Jun 17. Cereb Cortex. 2016. PMID: 26088972 Free PMC article.
-
Reversible Deactivation of Motor Cortex Reveals Functional Connectivity with Posterior Parietal Cortex in the Prosimian Galago (Otolemur garnettii).J Neurosci. 2015 Oct 21;35(42):14406-22. doi: 10.1523/JNEUROSCI.1468-15.2015. J Neurosci. 2015. PMID: 26490876 Free PMC article.
-
The dorsal stream of visual processing and action-specific domains in parietal and frontal cortex in primates.J Comp Neurol. 2023 Dec;531(18):1897-1908. doi: 10.1002/cne.25489. Epub 2023 Apr 28. J Comp Neurol. 2023. PMID: 37118872 Free PMC article. Review.
Cited by
-
The unique role of parietal cortex in action observation: Functional organization for communicative and manipulative actions.Neuroimage. 2021 Aug 15;237:118220. doi: 10.1016/j.neuroimage.2021.118220. Epub 2021 May 28. Neuroimage. 2021. PMID: 34058335 Free PMC article.
-
Neuroplasticity and Crossmodal Connectivity in the Normal, Healthy Brain.Psychol Neurosci. 2021 Sep;14(3):298-334. doi: 10.1037/pne0000258. Epub 2021 Jul 29. Psychol Neurosci. 2021. PMID: 36937077 Free PMC article.
-
Ipsilateral-Dominant Control of Limb Movements in Rodent Posterior Parietal Cortex.J Neurosci. 2019 Jan 16;39(3):485-502. doi: 10.1523/JNEUROSCI.1584-18.2018. Epub 2018 Nov 26. J Neurosci. 2019. PMID: 30478035 Free PMC article.
-
Cortical connections of the functional domain for climbing or running in posterior parietal cortex of galagos.J Comp Neurol. 2021 Jul 1;529(10):2789-2812. doi: 10.1002/cne.25123. Epub 2021 Mar 4. J Comp Neurol. 2021. PMID: 33550608 Free PMC article.
-
The self-in-the-world map emerged in the primate brain as a basis for Homo sapiens abilities.Dev Growth Differ. 2024 Aug;66(6):342-348. doi: 10.1111/dgd.12939. Epub 2024 Aug 8. Dev Growth Differ. 2024. PMID: 39113583 Free PMC article. Review.
References
-
- Andersen RA, Asanuma C, Cowan WM. Callosal and prefrontal associational projecting cell populations in area 7A of the macaque monkey: A study using retrogradely transported fluorescent dyes. J Comp Neurol. 1985;232:443–455. - PubMed
-
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–1259. - PubMed
-
- Bloch JI, Boyer DM. Grasping primate origins. Science. 2002;298:1606–1610. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

