Puerarin attenuates glucocorticoid-induced apoptosis of hFOB1.19 cells through the JNK- and Akt-mediated mitochondrial apoptotic pathways
- PMID: 26101183
- PMCID: PMC4501663
- DOI: 10.3892/ijmm.2015.2258
Puerarin attenuates glucocorticoid-induced apoptosis of hFOB1.19 cells through the JNK- and Akt-mediated mitochondrial apoptotic pathways
Abstract
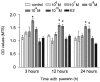
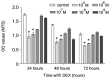
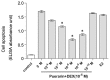
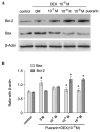
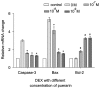
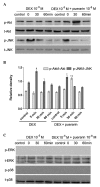
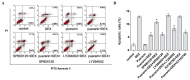
Puerarin is an active component of Pueraria lobata, which is a commonly used Chinese herbal medicine for the treatment of osteoporosis. The present study aimed to evaluate the osteoprotective effect of puerarin on glucocorticoid (GC)-induced apoptosis of osteoblasts in vitro. The effects of puerarin on dexamethasone (DEX)-induced cell apoptosis were assessed using enzyme-linked immunosorbent assay and a terminal deoxynucleotidyl transferase dUTP nick-end labeling assay, and found that the viability of hFOB1.19 cells was significantly increased following exposure to between 10(-6) and 10(-10) M puerarin, with a maximal anti-apoptotic effect at a concentration of 10(-8) M. In addition, compared with the control group, puerarin upregulated the transcription and protein levels of B-cell lymphoma-2 and downregulated B-cell-associated X protein in the hFOB1.19 cells. Puerarin attenuated the DEX-induced release of cytochrome c and cleavage of caspase-3, and treatment with puerarin inhibited the c-Jun N-terminal kinase (JNK) pathway and activated the phosphoinositide 3-kinase (PI3K)/Akt pathway in the hFOB1.19 cells. Furthermore, the Akt inhibitor, LY294002, partly eliminated the protective effect of puerarin on DEX-induced apoptosis, and puerarin combined with the JNK inhibitor, SP600125, suppressed DEX-induced apoptosis to a lesser extent than in the cells treated with SP600125 alone. These results suggested that the JNK and PI3K/Akt signaling pathways mediate the inhibitory effects of puerarin on apoptosis in the hFOB1.19 cells. In conclusion, puerarin prevented DEX-induced apoptosis of hFOB1.19 cells via inhibition of the JNK pathway and activation of the PI3K/Akt signaling pathway in the cells, dependent on the mitochondrial apoptotic pathway. These results support puerarin as a promising target in the treatment of GC-induced osteoporosis.
Figures



References
-
- Canalis E. Clinical review 83: Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996;81:3441–3447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

