Efficacy of Adjuvant Trastuzumab for Patients With Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer and Tumors ≤ 2 cm: A Meta-Analysis of the Randomized Trastuzumab Trials
- PMID: 26101239
- PMCID: PMC4534523
- DOI: 10.1200/JCO.2015.60.8620
Efficacy of Adjuvant Trastuzumab for Patients With Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer and Tumors ≤ 2 cm: A Meta-Analysis of the Randomized Trastuzumab Trials
Abstract
Purpose: We compared efficacy of trastuzumab versus no trastuzumab in patients with small (≤ 2 cm) human epidermal growth factor receptor 2 (HER2) -positive breast cancer treated in randomized trials.
Methods: A meta-analysis was conducted using data from five of the six adjuvant trastuzumab trials. Efficacy end points were disease-free survival (DFS) and overall survival (OS). Separate analyses were prospectively planned for hormone receptor (HR) -positive and HR-negative cohorts. Random effect models and Yusuf-Peto fixed effects models assessed the impact of heterogeneity on baseline hazards and treatment effects across studies. Peto-Pike cumulative incidence estimates were stratified by study and nodal status.
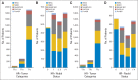
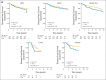
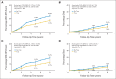
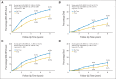
Results: Median follow-up time was 8 years. For 2,263 patients with HR-positive disease, 8-year cumulative incidence rates comparing trastuzumab versus no trastuzumab were 17.3% versus 24.3% (P < .001) for DFS and 7.8% versus 11.6% (P = .005) for OS, respectively; for 1,092 HR-positive patients with zero or one positive lymph nodes, results were 12.7% versus 19.4% (P = .005) for DFS and 5.3% versus 7.4% (P = .12) for OS, respectively. For 1,957 patients with HR-negative disease, 8-year cumulative incidence rates were 24.0% versus 33.4% (P < .001) for DFS and 12.4% versus 21.2% (P < .001) for OS, respectively; for 1,040 HR-negative patients with zero or one positive lymph nodes, results were 20.4% versus 26.3% (P = .05) for DFS and 8.2% versus 12.2% (P = .084) for OS, respectively.
Conclusion: Women with HER2-positive tumors ≤ 2 cm in the randomized trastuzumab trials derived substantial DFS and OS benefit from adjuvant trastuzumab. Trastuzumab-treated patients with HR-positive disease and ≤ one positive lymph node may be candidates for trials assessing less aggressive treatment approaches.
Trial registration: ClinicalTrials.gov NCT00004067 NCT00005970 NCT00045032 NCT00054587.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Reply to A. Oguz et al.J Clin Oncol. 2016 Feb 20;34(6):640-1. doi: 10.1200/JCO.2015.65.1034. Epub 2015 Dec 7. J Clin Oncol. 2016. PMID: 26644531 No abstract available.
-
Treatment of Lymph Node-Negative, Early-Stage HER2-Positive Breast Cancer.J Clin Oncol. 2016 Feb 20;34(6):639-40. doi: 10.1200/JCO.2015.63.8411. Epub 2015 Dec 7. J Clin Oncol. 2016. PMID: 26644534 No abstract available.
References
-
- American Cancer Society. What are the key statistics about breast cancer? http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-ke....
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
-
- Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 Study. 2008 San Antonio Breast Cancer Symposium; December 10-14, 2008; San Antonio, TX. (abstr 62)
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

