Phase II Trial Assessing the Ability of Neoadjuvant Chemotherapy With or Without Second-Look Surgery to Eliminate Measurable Disease for Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study
- PMID: 26101244
- PMCID: PMC4507465
- DOI: 10.1200/JCO.2014.59.5132
Phase II Trial Assessing the Ability of Neoadjuvant Chemotherapy With or Without Second-Look Surgery to Eliminate Measurable Disease for Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study
Abstract
Purpose: This phase II trial evaluated the effect of neoadjuvant chemotherapy with or without second-look surgery before craniospinal irradiation on response rates and survival outcomes in children with newly diagnosed non-germinomatous germ cell tumors.
Patients and methods: Induction chemotherapy consisted of six cycles of carboplatin/etoposide alternating with ifosfamide/etoposide. Patients demonstrating less than complete response after induction chemotherapy were encouraged to undergo second-look surgery. Patients who did not achieve complete response or partial response after chemotherapy with or without second-look surgery proceeded to high-dose chemotherapy with thiotepa and etoposide and autologous peripheral blood stem-cell rescue before craniospinal irradiation.
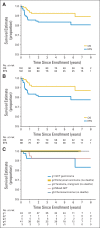
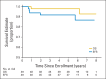
Results: The study included 102 patients treated between January 2004 and July 2008. Median age was 12 years, and 76% were male; 53.9% had pineal region masses, and 23.5% had suprasellar lesions. Sixty-nine percent of patients achieved complete response or partial response with neoadjuvant chemotherapy. At 5 years, event-free survival was 84% ± 4% (SE) and overall survival was 93% ± 3%. During the median follow-up of 5.1 years, 16 patients recurred or progressed, with seven deaths after relapse. No deaths were attributed to therapy-related toxicity. Relapse occurred at the site of primary disease in 10 patients, at a distant site in three patients, or both in one patient. In two patients, progression was detected by marker increase alone. Increased serum α-fetoprotein was a negative prognostic variable. Histologic subtype and increase of beta-human chorionic gonadotropin were not significantly correlated with worse outcomes.
Conclusion: Neoadjuvant chemotherapy with or without second-look surgery achieved high response rates contributing to excellent survival outcomes in children with newly diagnosed non-germinomatous germ cell tumors. This regimen should be included as a backbone for further studies.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures


References
-
- Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: A clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–455. - PubMed
-
- Hoffman HJ, Otsubo H, Hendrick EB, et al. Intracranial germ-cell tumors in children. J Neurosurg. 1991;74:545–551. - PubMed
-
- Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: A review. Oncologist. 2008;13:690–699. - PubMed
-
- Allen JC, DaRosso RC, Donahue B, et al. A phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer. 1994;74:940–944. - PubMed
-
- Buckner JC, Peethambaram PP, Smithson WA, et al. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol. 1999;17:933–940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

