SAMHD1 is a single-stranded nucleic acid binding protein with no active site-associated nuclease activity
- PMID: 26101257
- PMCID: PMC4513882
- DOI: 10.1093/nar/gkv633
SAMHD1 is a single-stranded nucleic acid binding protein with no active site-associated nuclease activity
Abstract
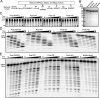
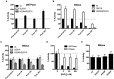
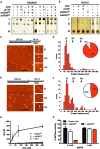
The HIV-1 restriction factor SAMHD1 is a tetrameric enzyme activated by guanine nucleotides with dNTP triphosphate hydrolase activity (dNTPase). In addition to this established activity, there have been a series of conflicting reports as to whether the enzyme also possesses single-stranded DNA and/or RNA 3'-5' exonuclease activity. SAMHD1 was purified using three chromatography steps, over which the DNase activity was largely separated from the dNTPase activity, but the RNase activity persisted. Surprisingly, we found that catalytic and nucleotide activator site mutants of SAMHD1 with no dNTPase activity retained the exonuclease activities. Thus, the exonuclease activity cannot be associated with any known dNTP binding site. Monomeric SAMHD1 was found to bind preferentially to single-stranded RNA, while the tetrameric form required for dNTPase action bound weakly. ssRNA binding, but not ssDNA, induces higher-order oligomeric states that are distinct from the tetrameric form that binds dNTPs. We conclude that the trace exonuclease activities detected in SAMHD1 preparations arise from persistent contaminants that co-purify with SAMHD1 and not from the HD active site. An in vivo model is suggested where SAMHD1 alternates between the mutually exclusive functions of ssRNA binding and dNTP hydrolysis depending on dNTP pool levels and the presence of viral ssRNA.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Hult K., Berglund P. Enzyme promiscuity: mechanism and applications. Trends Biotechnol. 2007;25:231–238. - PubMed
-
- Richardson C.C., Kornberg A. A deoxyribonucleic acid phosphatase-exonuclease from escherichia coli. I. Purification of the enzyme and characterization of the phosphatase activity. J. Biol. Chem. 1964;239:242–250. - PubMed
-
- Suck D. DNA recognition by DNase I. J. Mol. Recognit. 1994;7:65–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

