Post-translational environmental switch of RadA activity by extein-intein interactions in protein splicing
- PMID: 26101259
- PMCID: PMC4513877
- DOI: 10.1093/nar/gkv612
Post-translational environmental switch of RadA activity by extein-intein interactions in protein splicing
Abstract
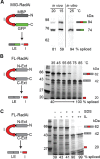
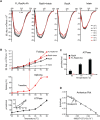
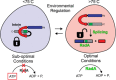
Post-translational control based on an environmentally sensitive intervening intein sequence is described. Inteins are invasive genetic elements that self-splice at the protein level from the flanking host protein, the exteins. Here we show in Escherichia coli and in vitro that splicing of the RadA intein located in the ATPase domain of the hyperthermophilic archaeon Pyrococcus horikoshii is strongly regulated by the native exteins, which lock the intein in an inactive state. High temperature or solution conditions can unlock the intein for full activity, as can remote extein point mutations. Notably, this splicing trap occurs through interactions between distant residues in the native exteins and the intein, in three-dimensional space. The exteins might thereby serve as an environmental sensor, releasing the intein for full activity only at optimal growth conditions for the native organism, while sparing ATP consumption under conditions of cold-shock. This partnership between the intein and its exteins, which implies coevolution of the parasitic intein and its host protein may provide a novel means of post-translational control.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

