Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers
- PMID: 26101265
- PMCID: PMC4493408
- DOI: 10.1084/jem.20142110
Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers
Abstract
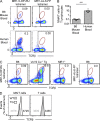
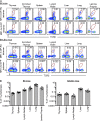
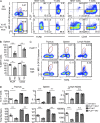
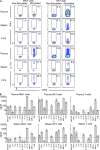
Studies on the biology of mucosal-associated invariant T cells (MAIT cells) in mice have been hampered by a lack of specific reagents. Using MR1-antigen (Ag) tetramers that specifically bind to the MR1-restricted MAIT T cell receptors (TCRs), we demonstrate that MAIT cells are detectable in a broad range of tissues in C57BL/6 and BALB/c mice. These cells include CD4(-)CD8(-), CD4(-)CD8(+), and CD4(+)CD8(-) subsets, and their frequency varies in a tissue- and strain-specific manner. Mouse MAIT cells have a CD44(hi)CD62L(lo) memory phenotype and produce high levels of IL-17A, whereas other cytokines, including IFN-γ, IL-4, IL-10, IL-13, and GM-CSF, are produced at low to moderate levels. Consistent with high IL-17A production, most MAIT cells express high levels of retinoic acid-related orphan receptor γt (RORγt), whereas RORγt(lo) MAIT cells predominantly express T-bet and produce IFN-γ. Most MAIT cells express the promyelocytic leukemia zinc finger (PLZF) transcription factor, and their development is largely PLZF dependent. These observations contrast with previous reports that MAIT cells from Vα19 TCR transgenic mice are PLZF(-) and express a naive CD44(lo) phenotype. Accordingly, MAIT cells from normal mice more closely resemble human MAIT cells than previously appreciated, and this provides the foundation for further investigations of these cells in health and disease.
© 2015 Rahimpour et al.
Figures



Similar articles
-
Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation.J Clin Invest. 2015 Nov 2;125(11):4171-85. doi: 10.1172/JCI82424. Epub 2015 Oct 12. J Clin Invest. 2015. PMID: 26524590 Free PMC article.
-
Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells.J Exp Med. 2013 Oct 21;210(11):2305-20. doi: 10.1084/jem.20130958. Epub 2013 Oct 7. J Exp Med. 2013. PMID: 24101382 Free PMC article.
-
Functional Heterogeneity and Antimycobacterial Effects of Mouse Mucosal-Associated Invariant T Cells Specific for Riboflavin Metabolites.J Immunol. 2015 Jul 15;195(2):587-601. doi: 10.4049/jimmunol.1402545. Epub 2015 Jun 10. J Immunol. 2015. PMID: 26063000 Free PMC article.
-
Mucosal-associated invariant T cells: unconventional development and function.Trends Immunol. 2011 May;32(5):212-8. doi: 10.1016/j.it.2011.02.005. Epub 2011 Apr 2. Trends Immunol. 2011. PMID: 21459674 Review.
-
MAIT, MR1, microbes and riboflavin: a paradigm for the co-evolution of invariant TCRs and restricting MHCI-like molecules?Immunogenetics. 2016 Aug;68(8):537-48. doi: 10.1007/s00251-016-0927-9. Epub 2016 Jul 8. Immunogenetics. 2016. PMID: 27393664 Review.
Cited by
-
Human MAIT-cell responses to Escherichia coli: activation, cytokine production, proliferation, and cytotoxicity.J Leukoc Biol. 2016 Jul;100(1):233-40. doi: 10.1189/jlb.4TA0815-391RR. Epub 2016 Mar 31. J Leukoc Biol. 2016. PMID: 27034405 Free PMC article.
-
The Study of Mucosal-Associated Invariant T Cells in Colon Cancer and Roles in Immune Activities.Onco Targets Ther. 2021 Nov 16;14:5263-5273. doi: 10.2147/OTT.S332822. eCollection 2021. Onco Targets Ther. 2021. PMID: 34815674 Free PMC article.
-
Mucosal-associated invariant T cells have therapeutic potential against ocular autoimmunity.Mucosal Immunol. 2022 Feb;15(2):351-361. doi: 10.1038/s41385-021-00469-5. Epub 2021 Nov 13. Mucosal Immunol. 2022. PMID: 34775490
-
Elucidating Specificity Opens a Window to the Complexity of Both the Innate and Adaptive Immune Systems.Viral Immunol. 2020 Apr;33(3):145-152. doi: 10.1089/vim.2019.0186. Viral Immunol. 2020. PMID: 32286183 Free PMC article.
-
Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation.J Clin Invest. 2015 Nov 2;125(11):4171-85. doi: 10.1172/JCI82424. Epub 2015 Oct 12. J Clin Invest. 2015. PMID: 26524590 Free PMC article.
References
-
- Dusseaux M., Martin E., Serriari N., Péguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E., and Lantz O.. 2011. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17–secreting T cells. Blood. 117:1250–1259. 10.1182/blood-2010-08-303339 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

