Definition of a Novel Pathway Centered on Lysophosphatidic Acid To Recruit Monocytes during the Resolution Phase of Tissue Inflammation
- PMID: 26101324
- PMCID: PMC4505961
- DOI: 10.4049/jimmunol.1500733
Definition of a Novel Pathway Centered on Lysophosphatidic Acid To Recruit Monocytes during the Resolution Phase of Tissue Inflammation
Abstract
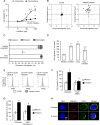
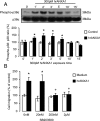
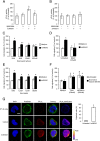
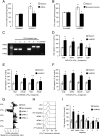
Blood-derived monocytes remove apoptotic cells and terminate inflammation in settings as diverse as atherosclerosis and Alzheimer's disease. They express high levels of the proresolving receptor ALX/FPR2, which is activated by the protein annexin A1 (ANXA1), found in high abundance in inflammatory exudates. Using primary human blood monocytes from healthy donors, we identified ANXA1 as a potent CD14(+)CD16(-) monocyte chemoattractant, acting via ALX/FPR2. Downstream signaling pathway analysis revealed the p38 MAPK-mediated activation of a calcium independent phospholipase A2 with resultant synthesis of lysophosphatidic acid (LPA) driving chemotaxis through LPA receptor 2 and actin cytoskeletal mobilization. In vivo experiments confirmed ANXA1 as an independent phospholipase A2-dependent monocyte recruiter; congruently, monocyte recruitment was significantly impaired during ongoing zymosan-induced inflammation in AnxA1(-/-) or alx/fpr2/3(-/-) mice. Using a dorsal air-pouch model, passive transfer of apoptotic neutrophils between AnxA1(-/-) and wild-type mice identified effete neutrophils as the primary source of soluble ANXA1 in inflammatory resolution. Together, these data elucidate a novel proresolving network centered on ANXA1 and LPA generation and identify previously unappreciated determinants of ANXA1 and ALX/FPR2 signaling in monocytes.
Copyright © 2015 The Authors.
Figures

Similar articles
-
Evidence for an anti-inflammatory loop centered on polymorphonuclear leukocyte formyl peptide receptor 2/lipoxin A4 receptor and operative in the inflamed microvasculature.J Immunol. 2011 Apr 15;186(8):4905-14. doi: 10.4049/jimmunol.1003145. Epub 2011 Mar 11. J Immunol. 2011. PMID: 21398608 Free PMC article.
-
Proresolving and tissue-protective actions of annexin A1-based cleavage-resistant peptides are mediated by formyl peptide receptor 2/lipoxin A4 receptor.J Immunol. 2013 Jun 15;190(12):6478-87. doi: 10.4049/jimmunol.1203000. Epub 2013 May 17. J Immunol. 2013. PMID: 23686496
-
Inflammation and cancer: role of annexin A1 and FPR2/ALX in proliferation and metastasis in human laryngeal squamous cell carcinoma.PLoS One. 2014 Dec 9;9(12):e111317. doi: 10.1371/journal.pone.0111317. eCollection 2014. PLoS One. 2014. PMID: 25490767 Free PMC article.
-
Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance.J Immunol Res. 2016;2016:8239258. doi: 10.1155/2016/8239258. Epub 2016 Jan 13. J Immunol Res. 2016. PMID: 26885535 Free PMC article. Review.
-
Annexin-A1: Therapeutic Potential in Microvascular Disease.Front Immunol. 2019 Apr 30;10:938. doi: 10.3389/fimmu.2019.00938. eCollection 2019. Front Immunol. 2019. PMID: 31114582 Free PMC article. Review.
Cited by
-
Phospholipid and Lipid Derivatives as Potential Neuroprotective Compounds.Molecules. 2018 Sep 5;23(9):2257. doi: 10.3390/molecules23092257. Molecules. 2018. PMID: 30189584 Free PMC article. Review.
-
Single-cell analysis of ovarian myeloid cells identifies aging associated changes in macrophages and signaling dynamics.bioRxiv [Preprint]. 2024 Jun 16:2024.06.13.598667. doi: 10.1101/2024.06.13.598667. bioRxiv. 2024. Update in: Biol Reprod. 2025 Aug 13;113(2):373-386. doi: 10.1093/biolre/ioaf122. PMID: 38915572 Free PMC article. Updated. Preprint.
-
CF monocyte-derived macrophages have an attenuated response to extracellular vesicles secreted by airway epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2021 Apr 1;320(4):L530-L544. doi: 10.1152/ajplung.00621.2020. Epub 2021 Jan 20. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33471607 Free PMC article.
-
Diacylglycerols and Lysophosphatidic Acid, Enriched on Lipoprotein(a), Contribute to Monocyte Inflammation.Arterioscler Thromb Vasc Biol. 2024 Mar;44(3):720-740. doi: 10.1161/ATVBAHA.123.319937. Epub 2024 Jan 25. Arterioscler Thromb Vasc Biol. 2024. PMID: 38269588 Free PMC article.
-
Cyclic AMP Regulates Key Features of Macrophages via PKA: Recruitment, Reprogramming and Efferocytosis.Cells. 2020 Jan 6;9(1):128. doi: 10.3390/cells9010128. Cells. 2020. PMID: 31935860 Free PMC article.
References
-
- Couzin-Frankel J. 2010. Inflammation bares a dark side. Science 330: 1621. - PubMed
-
- Serhan C. N., Savill J. 2005. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6: 1191–1197. - PubMed
-
- Rossi A. G., Sawatzky D. A., Walker A., Ward C., Sheldrake T. A., Riley N. A., Caldicott A., Martinez-Losa M., Walker T. R., Duffin R., et al. 2006. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12: 1056–1064. - PubMed
-
- Maslin C. L. V., Kedzierska K., Webster N. L., Muller W. A., Crowe S. M. 2005. Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection. Curr. HIV Res. 3: 303–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

