Selective Acetamidine-Based Nitric Oxide Synthase Inhibitors: Synthesis, Docking, and Biological Studies
- PMID: 26101565
- PMCID: PMC4468396
- DOI: 10.1021/acsmedchemlett.5b00149
Selective Acetamidine-Based Nitric Oxide Synthase Inhibitors: Synthesis, Docking, and Biological Studies
Abstract
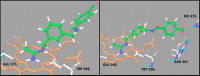
N-[(3-Aminomethyl)benzyl]acetamidine derivatives were synthesized and in vitro evaluated as inhibitors of the inducible isoform of nitric oxide synthase (iNOS). Because of the high potency of action and the excellent selectivity over the endothelial nitric oxide synthase (eNOS), compound 10 was ex vivo evaluated on isolated and perfused resistance arteries. The results confirm that compound 10 selectively inhibits the iNOS, without affecting the endothelial isoform. The outcome of the docking studies showed that the hydrophobic interaction is the driving force of the binding process, especially for iNOS, where the binding pocket is characterized by a significant lipophilic region.
Keywords: Acetamidine; inhibitor; molecular docking; nitric oxide synthases; selective; synthesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


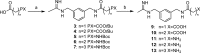
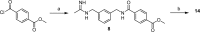


References
-
- Santolini J. The molecular mechanism of mammalian NO-synthases: A story of electrons and protons. J. Inorg. Biochem. 2011, 105, 127–141. - PubMed
-
- Zhou L.; Zhu D. Y. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 2009, 20, 223–230. - PubMed
-
- Pautz A.; Art J.; Hahn S.; Nowag S.; Voss C.; Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 23, 75–93. - PubMed
-
- Tinker A. C.; Wallace A. V. Selective inhibitors of inducible nitric oxide synthase: potential agents for the treatment of inflammatory diseases?. Curr. Top. Med. Chem. 2006, 6, 77–92. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

