Splicing variants of porcine synphilin-1
- PMID: 26101749
- PMCID: PMC4468357
- DOI: 10.1016/j.mgene.2015.04.005
Splicing variants of porcine synphilin-1
Abstract
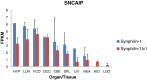
Parkinson's disease (PD), idiopathic and familial, is characterized by degradation of dopaminergic neurons and the presence of Lewy bodies (LB) in the substantia nigra. LBs contain aggregated proteins of which α-synuclein is the major component. The protein synphilin-1 interacts and colocalizes with α-synuclein in LBs. The aim of this study was to isolate and characterize porcine synphilin-1 and isoforms hereof with the future perspective to use the pig as a model for Parkinson's disease. The porcine SNCAIP cDNA was cloned by reverse transcriptase PCR. The spatial expression of SNCAIP mRNA was investigated by RNAseq. The presented work reports the molecular cloning and characterization of the porcine (Sus scrofa) synphilin-1 cDNA (SNCAIP) and three splice variants hereof. The porcine SNCAIP cDNA codes for a protein (synphilin-1) of 919 amino acids which shows a high similarity to human (90%) and to mouse (84%) synphilin-1. Three shorter transcript variants of the synphilin-1 gene were identified, all lacking one or more exons. SNCAIP transcripts were detected in most examined organs and tissues and the highest expression was found in brain tissues and lung. Conserved splicing variants and a novel splice form of synhilin-1 were found in this study. All synphilin-1 isoforms encoded by the identified transcript variants lack functional domains important for protein degradation.
Keywords: LB, Lewy body; Lewy body; ORF, open reading frame; Parkinson's disease; Pig; SNCAIP, α-synuclein interacting protein; Synphilin-1; α-Synuclein.
Figures
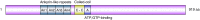


References
-
- Bjerre D., Madsen L.B., Bendixen C., Larsen K. Porcine parkin: molecular cloning of PARK2 cDNA, expression analysis, and identification of a splicing variant. Biochem. Biophys. Res. Commun. 2006;347:803–813. - PubMed
-
- Chung K.K., Zhang Y., Lim K.L., Tanaka Y., Huang H., Gao J., Ross C.A., Dawson V.L., Dawson T.M. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat. Med. 2001;7:1144–1150. - PubMed
-
- Crosiers D., Theuns J., Cras P., Van Broeckhoven C. Parkinson disease: insights in clinical, genetic and pathological features of monogenic disease subtypes. J. Chem. Neuroanat. 2011;42:131–141. - PubMed
-
- Dickson D.W., Braak H., Duda J.E., Duyckaerts C., Gasser T., Halliday G.M., Hardy J., Leverenz J.B., Del Tredici K., Wszolek Z.K., Litvan I. Neuropathological assessment of Parkinson disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. - PubMed
-
- Engelender S., Kaminsky Z., Guo X., Sharp A.H., Amaravi R.K., Kleiderlein J.J., Margolis R.L., Troncoso J.C., Lanahan A.A., Worley P.F., Dawson V.L., Dawson T.M., Ross C.A. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat. Genet. 1999;22:110–114. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

