Structure-activity relationships of bumetanide derivatives: correlation between diuretic activity in dogs and inhibition of the human NKCC2A transporter
- PMID: 26101812
- PMCID: PMC4562508
- DOI: 10.1111/bph.13231
Structure-activity relationships of bumetanide derivatives: correlation between diuretic activity in dogs and inhibition of the human NKCC2A transporter
Abstract
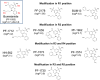
Background and purpose: The N-K-Cl cotransporters (NKCCs) mediate the coupled, electroneutral movement of Na+ , K+ and Cl- ions across cell membranes. There are two isoforms of this cation co-transporter, NKCC1 and NKCC2. NKCC2 is expressed primarily in the kidney and is the target of diuretics such as bumetanide. Bumetanide was discovered by screening ∼5000 3-amino-5-sulfamoylbenzoic acid derivatives, long before NKCC2 was identified in the kidney. Therefore, structure-activity studies on effects of bumetanide derivatives on NKCC2 are not available.
Experimental approach: In this study, the effect of a series of diuretically active bumetanide derivatives was investigated on human NKCC2 variant A (hNKCC2A) expressed in Xenopus laevis oocytes.
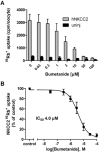
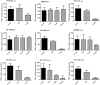
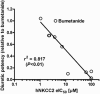
Key results: Bumetanide blocked hNKCC2A transport with an IC50 of 4 μM. There was good correlation between the diuretic potency of bumetanide and its derivatives in dogs and their inhibition of hNKCC2A (r2 = 0.817; P < 0.01). Replacement of the carboxylic group of bumetanide by a non-ionic residue, for example, an anilinomethyl group, decreased inhibition of hNKCC2A, indicating that an acidic group was required for transporter inhibition. Exchange of the phenoxy group of bumetanide for a 4-chloroanilino group or the sulfamoyl group by a methylsulfonyl group resulted in compounds with higher potency to inhibit hNKCC2A than bumetanide.
Conclusions and implications: The X. laevis oocyte expression system used in these experiments allowed analysis of the structural requirements that determine relative potency of loop diuretics on human NKCC2 splice variants, and may lead to the discovery of novel high-ceiling diuretics.
© 2015 The British Pharmacological Society.
Figures
Similar articles
-
The search for NKCC1-selective drugs for the treatment of epilepsy: Structure-function relationship of bumetanide and various bumetanide derivatives in inhibiting the human cation-chloride cotransporter NKCC1A.Epilepsy Behav. 2016 Jun;59:42-9. doi: 10.1016/j.yebeh.2016.03.021. Epub 2016 Apr 15. Epilepsy Behav. 2016. PMID: 27088517
-
Rat NKCC2/NKCC1 cotransporter selectivity for loop diuretic drugs.Naunyn Schmiedebergs Arch Pharmacol. 2002 Mar;365(3):193-9. doi: 10.1007/s00210-001-0521-y. Epub 2002 Feb 1. Naunyn Schmiedebergs Arch Pharmacol. 2002. PMID: 11882915
-
Bumepamine, a brain-permeant benzylamine derivative of bumetanide, does not inhibit NKCC1 but is more potent to enhance phenobarbital's anti-seizure efficacy.Neuropharmacology. 2018 Dec;143:186-204. doi: 10.1016/j.neuropharm.2018.09.025. Epub 2018 Sep 21. Neuropharmacology. 2018. PMID: 30248303
-
CNS pharmacology of NKCC1 inhibitors.Neuropharmacology. 2022 Mar 1;205:108910. doi: 10.1016/j.neuropharm.2021.108910. Epub 2021 Dec 6. Neuropharmacology. 2022. PMID: 34883135 Review.
-
[The loop diuretic bumetanide as a tool in physiology and pharmacology].Dtsch Tierarztl Wochenschr. 1992 Oct;99(10):412-5. Dtsch Tierarztl Wochenschr. 1992. PMID: 1425319 Review. German.
Cited by
-
Azosemide is more potent than bumetanide and various other loop diuretics to inhibit the sodium-potassium-chloride-cotransporter human variants hNKCC1A and hNKCC1B.Sci Rep. 2018 Jun 29;8(1):9877. doi: 10.1038/s41598-018-27995-w. Sci Rep. 2018. PMID: 29959396 Free PMC article.
-
The role of SLC12A family of cation-chloride cotransporters and drug discovery methodologies.J Pharm Anal. 2023 Dec;13(12):1471-1495. doi: 10.1016/j.jpha.2023.09.002. Epub 2023 Sep 9. J Pharm Anal. 2023. PMID: 38223443 Free PMC article. Review.
-
Ion transporter cascade, reactive astrogliosis and cerebrovascular diseases.Front Pharmacol. 2024 Apr 9;15:1374408. doi: 10.3389/fphar.2024.1374408. eCollection 2024. Front Pharmacol. 2024. PMID: 38659577 Free PMC article. Review.
-
Diuretics: a review of the pharmacology and effects on glucose homeostasis.Front Pharmacol. 2025 Mar 28;16:1513125. doi: 10.3389/fphar.2025.1513125. eCollection 2025. Front Pharmacol. 2025. PMID: 40223924 Free PMC article. Review.
-
Simultaneous determination of five diuretic drugs using quantitative analysis of multiple components by a single marker.BMC Chem. 2021 Jun 9;15(1):39. doi: 10.1186/s13065-021-00764-z. BMC Chem. 2021. PMID: 34108013 Free PMC article.
References
-
- Alvarez-Leefmans FJ. Intracellular chloride regulation. In: Sperelakis N, editor. Cell physiology Sourcebook. Fourth Edition. Essentials of Membrane Biophysics. London: Academic Press; 2012. pp. 221–259.
-
- Ben Ari Y. Blocking seizures with the diuretic bumetanide: promises and pitfalls. Epilepsia. 2012;53:394–396. - PubMed
-
- Burckhardt G. Drug transport by Organic Anion Transporters (OATs) Pharmacol Ther. 2012;136:106–130. - PubMed
-
- Carota I, Theilig F, Oppermann M, Kongsuphol P, Rosenauer A, Schreiber R, et al. Localization and functional characterization of the human NKCC2 isoforms. Acta Physiol (Oxf) 2010;199:327–338. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

