Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC)
- PMID: 26101915
- PMCID: PMC4673178
- DOI: 10.18632/oncotarget.4318
Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC)
Erratum in
-
Correction: Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC).Oncotarget. 2020 Mar 10;11(10):942. doi: 10.18632/oncotarget.27512. eCollection 2020 Mar 10. Oncotarget. 2020. PMID: 32206190 Free PMC article.
Abstract
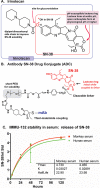
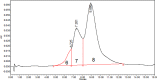
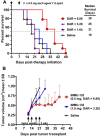
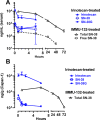
Trop-2 is a novel target for ADC therapy because of its high expression by many solid cancers. The rational development of IMMU-132 represents a paradigm shift as an ADC that binds a well-known moderately-cytotoxic drug, SN-38, to the anti-Trop-2 antibody. In vitro and in vivo studies show enhanced efficacy, while there is a gradual release of SN-38 that contributes to the overall effect. IMMU-132 is most efficacious at a high drug:antibody ratio (DAR) of 7.6:1, which does not affect binding and pharmacokinetics. It targets up to 136-fold more SN-38 to a human cancer xenograft than irinotecan, SN-38's prodrug. IMMU-132 delivers SN-38 in its most active, non-glucuronidated form, which may explain the lower frequency of severe diarrhea than with irinotecan. Thus, this ADC, carrying a moderately-toxic drug targeting Trop-2 represents a novel cancer therapeutic that is showing promising activity in patients with several metastatic cancer types, including triple-negative breast cancer, non-small-cell and small-cell lung cancers.
Keywords: SN-38; Trop-2; antibody-drug conjugate; solid cancers; triple-negative breast cancer.
Conflict of interest statement
All authors have employment and stock or stock options in Immunomedics, Inc.
Figures



References
-
- Basu A, Goldenberg DM, Stein R. The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7–3G11, is phosphorylated on serine 303. Int J Cancer. 1995;62:472–479. - PubMed
-
- Stein R, Chen S, Sharkey RM, Goldenberg DM. Murine monoclonal antibodies raised against human non-small cell carcinoma of the lung: specificity and tumor targeting. Cancer Res. 1990;50:1330–1336. - PubMed
-
- Stein R, Basu A, Chen S, Shih LB, Goldenberg DM. Specificity and properties of MAb RS7–3G11 and the antigen defined by this pancarcinoma monoclonal antibody. Int J Cancer. 1993;55:938–946. - PubMed
-
- Calabrese G, Crescenzi C, Morizio E, Palka G, Guerra E, Alberti S. Assignment of TACSTD1 (alias TROP1, M4S1) to human chromosome 2p21 and refinement of mapping of TACSTD2 (alias TROP2, M1S1) to human chromosome 1p32 by in situ hybridization. Cytogenet Cell Genet. 2001;92:164–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

