Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation
- PMID: 26102003
- PMCID: PMC4532582
- DOI: 10.1016/j.neuroscience.2015.05.081
Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation
Abstract
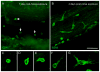
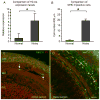
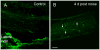
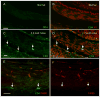
The immune response is an important component of the cochlear response to stress. As an important player in the cochlear immune system, the basilar membrane immune cells reside on the surface of the scala tympani side of the basilar membrane. At present, the immune cell properties in this region and their responses to stress are not well understood. Here, we investigated the functional role of these immune cells in the immune response to acoustic overstimulation. This study reveals that tissue macrophages are present in the entire length of the basilar membrane under steady-state conditions. Notably, these cells in the apical and the basal sections of the basilar membrane display distinct morphologies and immune protein expression patterns. Following acoustic trauma, monocytes infiltrate into the region of the basilar membrane, and the infiltrated cells transform into macrophages. While monocyte infiltration and transformation occur in both the apical and the basal sections of the basilar membrane, only the basal monocytes and macrophages display a marked increase in the expression of major histocompatibility complex (MHC) II and class II transactivator (CIITA), a MHC II production cofactor, suggesting the site-dependent activation of antigen-presenting function. Consistent with the increased expression of the antigen-presenting proteins, CD4(+) T cells, the antigen-presenting partner, infiltrate into the region of the basilar membrane where antigen-presenting proteins are upregulated. Further pathological analyses revealed that the basal section of the cochlea displays a greater level of sensory cell damage, which is spatially correlated with the region of antigen-presenting activity. Together, these results suggest that the antigen-presenting function of the mononuclear phagocyte population is activated in response to acoustic trauma, which could bridge the innate immune response to adaptive immunity.
Keywords: MHC II; antigen; cochlea; immunity; macrophage; noise.
Copyright © 2015 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Dynamic activation of basilar membrane macrophages in response to chronic sensory cell degeneration in aging mouse cochleae.Hear Res. 2017 Feb;344:125-134. doi: 10.1016/j.heares.2016.11.003. Epub 2016 Nov 9. Hear Res. 2017. PMID: 27837652 Free PMC article.
-
Toll-like receptor 4 modulates the cochlear immune response to acoustic injury.Cell Death Dis. 2016 Jun 2;7(6):e2245. doi: 10.1038/cddis.2016.156. Cell Death Dis. 2016. PMID: 27253409 Free PMC article.
-
SYK regulates macrophage MHC-II expression via activation of autophagy in response to oxidized LDL.Autophagy. 2015;11(5):785-95. doi: 10.1080/15548627.2015.1037061. Autophagy. 2015. PMID: 25946330 Free PMC article.
-
Human mononuclear phagocyte system reunited.Semin Cell Dev Biol. 2015 May;41:59-69. doi: 10.1016/j.semcdb.2015.05.004. Epub 2015 May 15. Semin Cell Dev Biol. 2015. PMID: 25986054 Review.
-
Immune recognition and effector function in subsets of CD4 T cells.Princess Takamatsu Symp. 1988;19:193-208. Princess Takamatsu Symp. 1988. PMID: 2908353 Review.
Cited by
-
Chronic suppurative otitis media causes macrophage-associated sensorineural hearing loss.J Neuroinflammation. 2022 Sep 12;19(1):224. doi: 10.1186/s12974-022-02585-w. J Neuroinflammation. 2022. PMID: 36096817 Free PMC article.
-
Multiplex immunohistochemistry reveals cochlear macrophage heterogeneity and local auditory nerve inflammation in cisplatin-induced hearing loss.Front Neurol. 2022 Oct 20;13:1015014. doi: 10.3389/fneur.2022.1015014. eCollection 2022. Front Neurol. 2022. PMID: 36341090 Free PMC article.
-
Macrophages in the Human Cochlea: Saviors or Predators-A Study Using Super-Resolution Immunohistochemistry.Front Immunol. 2018 Feb 13;9:223. doi: 10.3389/fimmu.2018.00223. eCollection 2018. Front Immunol. 2018. PMID: 29487598 Free PMC article.
-
Innate Immunity to Spiral Ganglion Neuron Loss: A Neuroprotective Role of Fractalkine Signaling in Injured Cochlea.Front Cell Neurosci. 2021 Aug 2;15:694292. doi: 10.3389/fncel.2021.694292. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34408629 Free PMC article. Review.
-
Immuno-surveillance and protection of the human cochlea.Front Neurol. 2024 May 16;15:1355785. doi: 10.3389/fneur.2024.1355785. eCollection 2024. Front Neurol. 2024. PMID: 38817543 Free PMC article.
References
-
- Bohne BA, Harding GW, Lee SC. Death pathways in noise-damaged outer hair cells. Hear Res. 2007;223:61–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

