Triel Bonds, π-Hole-π-Electrons Interactions in Complexes of Boron and Aluminium Trihalides and Trihydrides with Acetylene and Ethylene
- PMID: 26102066
- PMCID: PMC6272408
- DOI: 10.3390/molecules200611297
Triel Bonds, π-Hole-π-Electrons Interactions in Complexes of Boron and Aluminium Trihalides and Trihydrides with Acetylene and Ethylene
Abstract
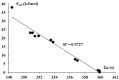
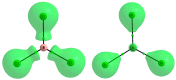
MP2/aug-cc-pVTZ calculations were performed on complexes of aluminium and boron trihydrides and trihalides with acetylene and ethylene. These complexes are linked through triel bonds where the triel center (B or Al) is characterized by the Lewis acid properties through its π-hole region while π-electrons of C2H2 or C2H4 molecule play the role of the Lewis base. Some of these interactions possess characteristics of covalent bonds, i.e., the Al-π-electrons links as well as the interaction in the BH3-C2H2 complex. The triel-π-electrons interactions are classified sometimes as the 3c-2e bonds. In the case of boron trihydrides, these interactions are often the preliminary stages of the hydroboration reaction. The Quantum Theory of "Atoms in Molecules" as well as the Natural Bond Orbitals approach are applied here to characterize the π-hole-π-electrons interactions.
Keywords: Natural Bond Orbitals approach; Quantum Theory of “Atoms in Molecules”; boron and aluminium Lewis acid centres; triel bond; π-hole.
Conflict of interest statement
The author declares no conflict of interest.
Figures






References
-
- Jeffrey G.A., Saenger W. Hydrogen Bonding in Biological Structures. Springer-Verlag; Berlin, Germany: 1991.
-
- Jeffrey G.A. An Introduction to Hydrogen Bonding. Oxford University Press; New York, NY, USA: 1997.
-
- Desiraju G.R., Steiner T. The Weak Hydrogen Bond in Structural Chemistry and Biology. Oxford University Press; New York, NY, USA: 1999.
-
- Kollman P. A General analysis of noncovalent intermolecular interactions. J. Am. Chem. Soc. 1977;99:4875–4894. doi: 10.1021/ja00457a002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

