Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota
- PMID: 26102296
- PMCID: PMC4557287
- DOI: 10.1038/ncomms8489
Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota
Erratum in
-
Corrigendum: Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota.Nat Commun. 2017 Jul 11;8:16130. doi: 10.1038/ncomms16130. Nat Commun. 2017. PMID: 28695905 Free PMC article.
Abstract
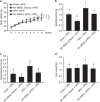
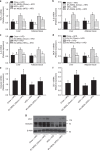
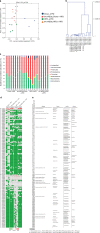
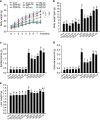
Obesity is associated with low-grade chronic inflammation and intestinal dysbiosis. Ganoderma lucidum is a medicinal mushroom used in traditional Chinese medicine with putative anti-diabetic effects. Here, we show that a water extract of Ganoderma lucidum mycelium (WEGL) reduces body weight, inflammation and insulin resistance in mice fed a high-fat diet (HFD). Our data indicate that WEGL not only reverses HFD-induced gut dysbiosis-as indicated by the decreased Firmicutes-to-Bacteroidetes ratios and endotoxin-bearing Proteobacteria levels-but also maintains intestinal barrier integrity and reduces metabolic endotoxemia. The anti-obesity and microbiota-modulating effects are transmissible via horizontal faeces transfer from WEGL-treated mice to HFD-fed mice. We further show that high molecular weight polysaccharides (>300 kDa) isolated from the WEGL extract produce similar anti-obesity and microbiota-modulating effects. Our results indicate that G. lucidum and its high molecular weight polysaccharides may be used as prebiotic agents to prevent gut dysbiosis and obesity-related metabolic disorders in obese individuals.
Conflict of interest statement
YFK is President of Chang Gung Biotechnology; JDY is Chairman of the Board of Chang Gung Biotechnology. The other authors declare no competing financial interests.
Figures
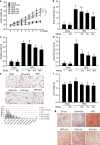
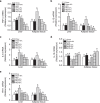
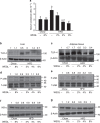
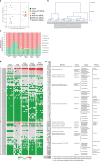
Comment in
-
Obesity: Medicinal mushroom reduces obesity by modulating microbiota.Nat Rev Endocrinol. 2015 Sep;11(9):504. doi: 10.1038/nrendo.2015.114. Epub 2015 Jul 14. Nat Rev Endocrinol. 2015. PMID: 26170024 No abstract available.
-
Gut microbiota: Ganoderma lucidum, a new prebiotic agent to treat obesity?Nat Rev Gastroenterol Hepatol. 2015 Oct;12(10):553-4. doi: 10.1038/nrgastro.2015.137. Epub 2015 Aug 18. Nat Rev Gastroenterol Hepatol. 2015. PMID: 26284561
References
-
- Stone R. Biochemistry. Lifting the veil on traditional Chinese medicine. Science 319, 709–710 (2008). - PubMed
-
- Tang J. L., Liu B. Y. & Ma K. W. Traditional Chinese medicine. Lancet 372, 1938–1940 (2008). - PubMed
-
- El Enshasy H. A. & Hatti-Kaul R. Mushroom immunomodulators: unique molecules with unlimited applications. Trends Biotechnol. 31, 668–677 (2013). - PubMed
-
- Wasser S. P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 89, 1323–1332 (2011). - PubMed
-
- Sanodiya B. S., Thakur G. S., Baghel R. K., Prasad G. B. & Bisen P. S. Ganoderma lucidum: a potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 10, 717–742 (2009). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

