Activity-Based Probe for N-Acylethanolamine Acid Amidase
- PMID: 26102511
- PMCID: PMC4575644
- DOI: 10.1021/acschembio.5b00197
Activity-Based Probe for N-Acylethanolamine Acid Amidase
Abstract
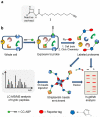
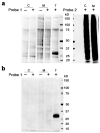
N-Acylethanolamine acid amidase (NAAA) is a lysosomal cysteine hydrolase involved in the degradation of saturated and monounsaturated fatty acid ethanolamides (FAEs), a family of endogenous lipid signaling molecules that includes oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). Among the reported NAAA inhibitors, α-amino-β-lactone (3-aminooxetan-2-one) derivatives have been shown to prevent FAE hydrolysis in innate-immune and neural cells and to reduce reactions to inflammatory stimuli. Recently, we disclosed two potent and selective NAAA inhibitors, the compounds ARN077 (5-phenylpentyl-N-[(2S,3R)-2-methyl-4-oxo-oxetan-3-yl]carbamate) and ARN726 (4-cyclohexylbutyl-N-[(S)-2-oxoazetidin-3-yl]carbamate). The former is active in vivo by topical administration in rodent models of hyperalgesia and allodynia, while the latter exerts systemic anti-inflammatory effects in mouse models of lung inflammation. In the present study, we designed and validated a derivative of ARN726 as the first activity-based protein profiling (ABPP) probe for the in vivo detection of NAAA. The newly synthesized molecule 1 is an effective in vitro and in vivo click-chemistry activity based probe (ABP), which is able to capture the catalytically active form of NAAA in Human Embryonic Kidney 293 (HEK293) cells overexpressing human NAAA as well as in rat lung tissue. Competitive ABPP with 1 confirmed that ARN726 and ARN077 inhibit NAAA in vitro and in vivo. Compound 1 is a useful new tool to identify activated NAAA both in vitro and in vivo and to investigate the physiological and pathological roles of this enzyme.
Figures
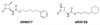

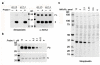

Similar articles
-
Synthesis and structure-activity relationship (SAR) of 2-methyl-4-oxo-3-oxetanylcarbamic acid esters, a class of potent N-acylethanolamine acid amidase (NAAA) inhibitors.J Med Chem. 2013 Sep 12;56(17):6917-34. doi: 10.1021/jm400739u. Epub 2013 Aug 30. J Med Chem. 2013. PMID: 23991897
-
Antinociceptive effects of the N-acylethanolamine acid amidase inhibitor ARN077 in rodent pain models.Pain. 2013 Mar;154(3):350-360. doi: 10.1016/j.pain.2012.10.018. Epub 2012 Nov 2. Pain. 2013. PMID: 23218523 Free PMC article.
-
Advances in the discovery of N-acylethanolamine acid amidase inhibitors.Pharmacol Res. 2014 Aug;86:11-7. doi: 10.1016/j.phrs.2014.04.011. Epub 2014 May 4. Pharmacol Res. 2014. PMID: 24798679 Free PMC article. Review.
-
An Important Role for N-Acylethanolamine Acid Amidase in the Complete Freund's Adjuvant Rat Model of Arthritis.J Pharmacol Exp Ther. 2016 Mar;356(3):656-63. doi: 10.1124/jpet.115.230516. Epub 2016 Jan 14. J Pharmacol Exp Ther. 2016. PMID: 26769918
-
N-Acylethanolamine Acid Amidase (NAAA): Structure, Function, and Inhibition.J Med Chem. 2020 Jul 23;63(14):7475-7490. doi: 10.1021/acs.jmedchem.0c00191. Epub 2020 Mar 26. J Med Chem. 2020. PMID: 32191459 Review.
Cited by
-
Second-Generation Non-Covalent NAAA Inhibitors are Protective in a Model of Multiple Sclerosis.Angew Chem Int Ed Engl. 2016 Sep 5;55(37):11193-11197. doi: 10.1002/anie.201603746. Epub 2016 Jul 12. Angew Chem Int Ed Engl. 2016. PMID: 27404798 Free PMC article.
-
Discovery and SAR Evolution of Pyrazole Azabicyclo[3.2.1]octane Sulfonamides as a Novel Class of Non-Covalent N-Acylethanolamine-Hydrolyzing Acid Amidase (NAAA) Inhibitors for Oral Administration.J Med Chem. 2021 Sep 23;64(18):13327-13355. doi: 10.1021/acs.jmedchem.1c00575. Epub 2021 Sep 1. J Med Chem. 2021. PMID: 34469137 Free PMC article.
-
Preparation and In Vivo Use of an Activity-based Probe for N-acylethanolamine Acid Amidase.J Vis Exp. 2016 Nov 23;(117):54652. doi: 10.3791/54652. J Vis Exp. 2016. PMID: 27911411 Free PMC article.
-
Chemical proteomics approaches for identifying the cellular targets of natural products.Nat Prod Rep. 2016 May 4;33(5):681-708. doi: 10.1039/c6np00001k. Nat Prod Rep. 2016. PMID: 27098809 Free PMC article. Review.
-
Synthesis, anti-inflammatory, bactericidal activities and docking studies of novel 1,2,3-triazoles derived from ibuprofen using click chemistry.Springerplus. 2016 Apr 11;5:423. doi: 10.1186/s40064-016-2052-5. eCollection 2016. Springerplus. 2016. PMID: 27104111 Free PMC article.
References
-
- Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J. Biol. Chem. 2005;280:11082–11092. - PubMed
-
- Tsuboi K, Takezaki N, Ueda N. The N-acylethanolamine-hydrolyzing acid amidase (NAAA) Chem. Biodivers. 2007;4:1914–1925. - PubMed
-
- Ueda N, Tsuboi K, Uyama T. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA) Prog. Lipid. Res. 2010;49:299–315. - PubMed
-
- Rossocha M, Schultz-Heienbrok R, von Moeller H, Coleman JP, Saenger W. Conjugated bile acid hydrolase is a tetrameric N-terminal thiol hydrolase with specific recognition of its cholyl but not of its tauryl product. Biochemistry. 2005;44:5739–5748. - PubMed
-
- Zhao LY, Tsuboi K, Okamoto Y, Nagahata S, Ueda N. Proteolytic activation and glycosylation of N-acylethanolamine-hydrolyzing acid amidase, a lysosomal enzyme involved in the endocannabinoid metabolism. Biochim. Biophys. Acta. 2007;1771:1397–1405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

