Exosomes and Their Role in the Life Cycle and Pathogenesis of RNA Viruses
- PMID: 26102580
- PMCID: PMC4488737
- DOI: 10.3390/v7062770
Exosomes and Their Role in the Life Cycle and Pathogenesis of RNA Viruses
Abstract
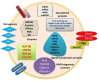
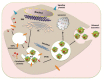
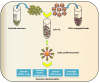
Exosomes are membrane-enclosed vesicles actively released into the extracellular space, whose content reflect the physiological/pathological state of the cells they originate from. These vesicles participate in cell-to-cell communication and transfer of biologically active proteins, lipids, and RNAs. Their role in viral infections is just beginning to be appreciated. RNA viruses are an important class of pathogens and affect millions of people worldwide. Recent studies on Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV), human T-cell lymphotropic virus (HTLV), and Dengue Virus (DENV) have demonstrated that exosomes released from infected cells harbor and deliver many regulatory factors including viral RNA and proteins, viral and cellular miRNA, and other host functional genetic elements to neighboring cells, helping to establish productive infections and modulating cellular responses. Exosomes can either spread or limit an infection depending on the type of pathogen and target cells, and can be exploited as candidates for development of antiviral or vaccine treatments. This review summarizes recent progress made in understanding the role of exosomes in RNA virus infections with an emphasis on their potential contribution to pathogenesis.
Keywords: RNA virus; exosomes; infection; miRNA; microvesicles; pathogenesis.
Figures

References
-
- Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262:9412–9420. - PubMed
-
- Harding C., Heuser J., Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: Demonstration of a pathway for receptor shedding. Eur. J. Cell Biol. 1984;35:256–263. - PubMed
-
- Thery C., Regnault A., Garin J., Wolfers J., Zitvogel L., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

