Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes
- PMID: 26102992
- PMCID: PMC4489430
- DOI: 10.1016/j.biomaterials.2015.06.009
Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes
Abstract
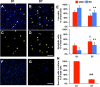
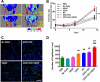
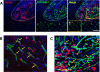
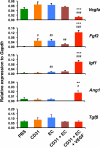
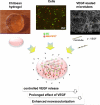
Various stem cells and their progeny have been used therapeutically for vascular regeneration. One of the major hurdles for cell-based therapy is low cell retention in vivo, and to improve cell survival several biomaterials have been used to encapsulate cells before transplantation. Vascular regeneration involves new blood vessel formation which consists of two processes, vasculogenesis and angiogenesis. While embryonic stem cell (ESC)-derived endothelial cells (ESC-ECs) have clearer vasculogenic potency, adult cells exert their effects mainly through paracrine angiogenic activities. While these two cells have seemingly complementary advantages, there have not been any studies to date combining these two cell types for vascular regeneration. We have developed a novel chitosan-based hydrogel construct that encapsulates both CD31-expressing BM-mononuclear cells (BM-CD31(+) cells) and ESC-ECs, and is loaded with VEGF-releasing microtubes. This cell construct showed high cell survival and minimal cytotoxicity in vitro. When implanted into a mouse model of hindlimb ischemia, it induced robust cell retention, neovascularization through vasculogenesis and angiogenesis, and efficiently induced recovery of blood flow in ischemic hindlimbs. This chitosan-based hydrogel encapsulating mixed adult and embryonic cell derivatives and containing VEGF can serve as a novel platform for treating various cardiovascular diseases.
Keywords: CD31(+) cells; Chitosan hydrogel; Embryonic stem cells; Lipid microtubes; Vascular regeneration.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Kinoshita M, Fujita Y, Katayama M, Baba R, Shibakawa M, Yoshikawa K, et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis. 2012;224(2):440–5. - PubMed
-
- Powell RJ. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. Journal of vascular surgery. 2012;56(1):264–6. - PubMed
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

