Activation of EP4 receptors prevents endotoxin-induced neutrophil infiltration into the airways and enhances microvascular barrier function
- PMID: 26103450
- PMCID: PMC4562507
- DOI: 10.1111/bph.13229
Activation of EP4 receptors prevents endotoxin-induced neutrophil infiltration into the airways and enhances microvascular barrier function
Abstract
Background and purpose: Pulmonary vascular dysfunction is a key event in acute lung injury. We recently demonstrated that PGE2 , via activation of E-prostanoid (EP)4 receptors, strongly enhances microvascular barrier function in vitro. The aim of this study was to investigate the beneficial effects of concomitant EP4 receptor activation in murine models of acute pulmonary inflammation.
Experimental approach: Pulmonary inflammation in male BALB/c mice was induced by LPS (20 μg per mouse intranasally) or oleic acid (0.15 μL·g-1 , i.v. ). In-vitro, endothelial barrier function was determined by measuring electrical impedance.
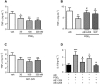
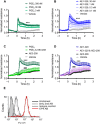
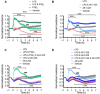
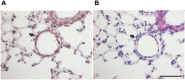
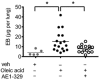
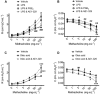
Key results: PGE2 activation of EP4 receptors reduced neutrophil infiltration, pulmonary vascular leakage and TNF-α concentration in bronchoalveolar lavage fluid from LPS-induced pulmonary inflammation. Similarly, pulmonary vascular hyperpermeability induced by oleic acid was counteracted by EP4 receptor activation. In lung function assays, the EP4 agonist ONO AE1-329 restored the increased resistance and reduced compliance upon methacholine challenge in mice treated with LPS or oleic acid. In agreement with these findings, EP4 receptor activation increased the in vitro vascular barrier function of human and mouse pulmonary microvascular endothelial cells and diminished the barrier disruption induced by LPS. The EP2 agonist ONO AE1-259 likewise reversed LPS-induced lung dysfunction without enhancing vascular barrier function.
Conclusion and implications: Our results show that activation of the EP4 receptor strengthens the microvascular barrier function and thereby ameliorates the pathology of acute lung inflammation, including neutrophil infiltration, vascular oedema formation and airway dysfunction. This suggests a potential benefit for EP4 agonists in acute pulmonary inflammation.
© 2015 The British Pharmacological Society.
Figures



Similar articles
-
The EP1/EP3 receptor agonist 17-pt-PGE2 acts as an EP4 receptor agonist on endothelial barrier function and in a model of LPS-induced pulmonary inflammation.Vascul Pharmacol. 2016 Dec;87:180-189. doi: 10.1016/j.vph.2016.09.008. Epub 2016 Sep 21. Vascul Pharmacol. 2016. PMID: 27664754 Free PMC article.
-
Endothelial E-type prostanoid 4 receptors promote barrier function and inhibit neutrophil trafficking.J Allergy Clin Immunol. 2013 Feb;131(2):532-40.e1-2. doi: 10.1016/j.jaci.2012.05.008. Epub 2012 Jun 15. J Allergy Clin Immunol. 2013. PMID: 22704539
-
Differential expression of E-type prostanoid receptors 2 and 4 in microglia stimulated with lipopolysaccharide.J Neuroinflammation. 2017 Jan 5;14(1):3. doi: 10.1186/s12974-016-0780-7. J Neuroinflammation. 2017. PMID: 28086956 Free PMC article.
-
Cooperativity of E-prostanoid receptor subtypes in regulating signaling and growth inhibition in human airway smooth muscle.FASEB J. 2019 Apr;33(4):4780-4789. doi: 10.1096/fj.201801959R. Epub 2019 Jan 2. FASEB J. 2019. PMID: 30601680 Free PMC article.
-
[Cooperation of two subtypes of PGE2 receptor, Gi coupled EP3 and Gs coupled EP2 or EP4 subtype].Yakugaku Zasshi. 2003 Oct;123(10):837-43. doi: 10.1248/yakushi.123.837. Yakugaku Zasshi. 2003. PMID: 14577329 Review. Japanese.
Cited by
-
The EP1/EP3 receptor agonist 17-pt-PGE2 acts as an EP4 receptor agonist on endothelial barrier function and in a model of LPS-induced pulmonary inflammation.Vascul Pharmacol. 2016 Dec;87:180-189. doi: 10.1016/j.vph.2016.09.008. Epub 2016 Sep 21. Vascul Pharmacol. 2016. PMID: 27664754 Free PMC article.
-
Thromboxane A2 exacerbates acute lung injury via promoting edema formation.Sci Rep. 2016 Aug 26;6:32109. doi: 10.1038/srep32109. Sci Rep. 2016. PMID: 27562142 Free PMC article.
-
PGE2 Produced by Exogenous MSCs Promotes Immunoregulation in ARDS Induced by Highly Pathogenic Influenza A through Activation of the Wnt-β-Catenin Signaling Pathway.Int J Mol Sci. 2023 Apr 14;24(8):7299. doi: 10.3390/ijms24087299. Int J Mol Sci. 2023. PMID: 37108459 Free PMC article. Review.
-
Protectin DX promotes the inflammatory resolution via activating COX-2/L-PGDS-PGD2 and DP1 receptor in acute respiratory distress syndrome.Int Immunopharmacol. 2022 Jan;102:108348. doi: 10.1016/j.intimp.2021.108348. Epub 2021 Nov 10. Int Immunopharmacol. 2022. PMID: 34920958 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. CIX. Differences and Similarities between Human and Rodent Prostaglandin E2 Receptors (EP1-4) and Prostacyclin Receptor (IP): Specific Roles in Pathophysiologic Conditions.Pharmacol Rev. 2020 Oct;72(4):910-968. doi: 10.1124/pr.120.019331. Pharmacol Rev. 2020. PMID: 32962984 Free PMC article.
References
-
- Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. - PubMed
-
- Aggarwal S, Moodley YP, Thompson PJ, Misso NL. Prostaglandin E2 and cysteinyl leukotriene concentrations in sputum: association with asthma severity and eosinophilic inflammation. Clin Exp Allergy. 2010;40:85–93. - PubMed
-
- Amann R, Schuligoi R, Peskar BA. Eicosanoid release in the endotoxin-primed isolated perfused rat lung and its pharmacological modification. Inflamm Res. 1999;48:632–636. - PubMed
-
- Benyahia C, Gomez I, Kanyinda L, Boukais K, Danel C, Leseche G, et al. PGE(2) receptor (EP(4)) agonists: potent dilators of human bronchi and future asthma therapy? Pulm Pharmacol Ther. 2012;25:115–118. - PubMed
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

