Alpha-2-Macroglobulin Is Acutely Sensitive to Freezing and Lyophilization: Implications for Structural and Functional Studies
- PMID: 26103636
- PMCID: PMC4477937
- DOI: 10.1371/journal.pone.0130036
Alpha-2-Macroglobulin Is Acutely Sensitive to Freezing and Lyophilization: Implications for Structural and Functional Studies
Abstract
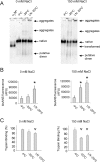
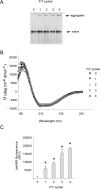
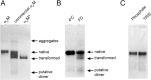
Alpha-2-macroglobulin is an abundant secreted protein that is of particular interest because of its diverse ligand binding profile and multifunctional nature, which includes roles as a protease inhibitor and as a molecular chaperone. The activities of alpha-2-macroglobulin are typically dependent on whether its conformation is native or transformed (i.e. adopts a more compact conformation after interactions with proteases or small nucleophiles), and are also influenced by dissociation of the native alpha-2-macroglobulin tetramer into stable dimers. Alpha-2-macroglobulin is predominately present as the native tetramer in vivo; once purified from human blood plasma, however, alpha-2-macroglobulin can undergo a number of conformational changes during storage, including transformation, aggregation or dissociation. We demonstrate that, particularly in the presence of sodium chloride or amine containing compounds, freezing and/or lyophilization of alpha-2-macroglobulin induces conformational changes with functional consequences. These conformational changes in alpha-2-macroglobulin are not always detected by standard native polyacrylamide gel electrophoresis, but can be measured using bisANS fluorescence assays. Increased surface hydrophobicity of alpha-2-macroglobulin, as assessed by bisANS fluorescence measurements, is accompanied by (i) reduced trypsin binding activity, (ii) increased chaperone activity, and (iii) increased binding to the surfaces of SH-SY5Y neurons, in part, via lipoprotein receptors. We show that sucrose (but not glycine) effectively protects native alpha-2-macroglobulin from denaturation during freezing and/or lyophilization, thereby providing a reproducible method for the handling and long-term storage of this protein.
Conflict of interest statement
Figures




Similar articles
-
The conformational state of human alpha 2-macroglobulin influences its dissociation into half-molecules by sodium thiocyanate.Arch Biochem Biophys. 1996 Sep 1;333(1):35-41. doi: 10.1006/abbi.1996.0361. Arch Biochem Biophys. 1996. PMID: 8806751
-
Conformational state and receptor recognition of the C-terminal domain of human alpha(2)-macroglobulin after dissociation into half-molecules.Clin Chim Acta. 2001 Aug 20;310(2):157-63. doi: 10.1016/s0009-8981(01)00572-1. Clin Chim Acta. 2001. PMID: 11498081
-
Structural Investigations of Human A2M Identify a Hollow Native Conformation That Underlies Its Distinctive Protease-Trapping Mechanism.Mol Cell Proteomics. 2021;20:100090. doi: 10.1016/j.mcpro.2021.100090. Epub 2021 May 6. Mol Cell Proteomics. 2021. PMID: 33964423 Free PMC article.
-
Alpha 2-macroglobulin and pregnancy zone protein. Serum levels, alpha 2-macroglobulin receptors, cellular synthesis and aspects of function in relation to immunology.Dan Med Bull. 1993 Sep;40(4):409-46. Dan Med Bull. 1993. PMID: 7693397 Review.
-
α-2-Macroglobulin: a physiological guardian.J Cell Physiol. 2013 Aug;228(8):1665-75. doi: 10.1002/jcp.24266. J Cell Physiol. 2013. PMID: 23086799 Review.
Cited by
-
Frozen fresh blood plasma preserves the functionality of native human α2-macroglobulin.Sci Rep. 2023 Mar 20;13(1):4579. doi: 10.1038/s41598-023-31800-8. Sci Rep. 2023. PMID: 36941303 Free PMC article.
-
Human pregnancy zone protein stabilizes misfolded proteins including preeclampsia- and Alzheimer's-associated amyloid beta peptide.Proc Natl Acad Sci U S A. 2019 Mar 26;116(13):6101-6110. doi: 10.1073/pnas.1817298116. Epub 2019 Mar 8. Proc Natl Acad Sci U S A. 2019. PMID: 30850528 Free PMC article.
-
Recent cryogenic electron microscopy structures of human A2M may not be representative of the native protein.Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2210218119. doi: 10.1073/pnas.2210218119. Epub 2022 Aug 16. Proc Natl Acad Sci U S A. 2022. PMID: 35972981 Free PMC article. No abstract available.
-
"Thromboinflammation in COVID-19: can α2-macroglobulin help to control the fire?": Comment from Seitz et al.J Thromb Haemost. 2023 Mar;21(3):704-705. doi: 10.1016/j.jtha.2023.01.005. J Thromb Haemost. 2023. PMID: 36858795 Free PMC article. No abstract available.
-
Molecular form and concentration of serum α2-macroglobulin in diabetes.Sci Rep. 2019 Sep 10;9(1):12927. doi: 10.1038/s41598-019-49144-7. Sci Rep. 2019. PMID: 31506491 Free PMC article.
References
-
- Imber MJ, Pizzo SV. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981;256(15):8134–9. Epub 1981/08/10. . - PubMed
-
- Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265(29):17401–4. Epub 1990/10/15. . - PubMed
-
- Fabrizi C, Businaro R, Lauro GM, Fumagalli L. Role of alpha2-macroglobulin in regulating amyloid beta-protein neurotoxicity: protective or detrimental factor? J Neurochem. 2001;78(2):406–12. Epub 2001/07/20. . - PubMed
-
- Narita M, Holtzman DM, Schwartz AL, Bu G. Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J Neurochem. 1997;69(5):1904–11. Epub 1998/02/12. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

