Apelin promotes diabetic nephropathy by inducing podocyte dysfunction via inhibiting proteasome activities
- PMID: 26103809
- PMCID: PMC4568931
- DOI: 10.1111/jcmm.12619
Apelin promotes diabetic nephropathy by inducing podocyte dysfunction via inhibiting proteasome activities
Abstract
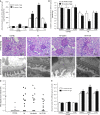
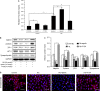
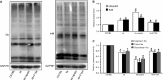
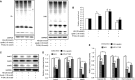
Podocyte injuries are associated with progression of diabetic nephropathy (DN). Apelin, an adipocyte-derived peptide, has been reported to be a promoting factor for DN. In this study, we aim to determine whether apelin promotes progression of DN by inducing podocyte dysfunction. kk-Ay mice were used as models for DN. Apelin and its antagonist, F13A were intraperitoneally administered for 4 weeks, respectively. Renal function and foot process proteins were analysed to evaluate the effects of apelin on kk-Ay mice and podocytes. Apelin increased albuminuria and decreased podocyte foot process proteins expression in kk-Ay mice, which is consistent with the results that apelin receptor (APLNR) levels increased in glomeruli of patients or mice with DN. In cultured podocytes, high glucose increased APLNR expression and apelin administration was associated with increased permeability and decreased foot process proteins levels. All these dysfunctions were associated with decreased 26S proteasome activities and increased polyubiquitinated proteins in both kk-Ay mice and cultured podocytes, as demonstrated by 26S proteasome activation with cyclic adenosine monophosphate (cAMP) or oleuropein. These effects seemed to be related to endoplasmic reticulum (ER) stress, as apelin increased C/EBP homologous protein (CHOP) and peiFα levels while cAMP or oleuropein reduced it in high glucose and apelin treated podocytes. These results suggest that apelin induces podocyte dysfunction in DN through ER stress which was induced by decreased proteasome activities in podocytes.
Keywords: APLNR; ER stress; apelin; diabetic nephropathy; podocyte; proteasome.
© 2015 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



Similar articles
-
Apelin involved in progression of diabetic nephropathy by inhibiting autophagy in podocytes.Cell Death Dis. 2017 Aug 24;8(8):e3006. doi: 10.1038/cddis.2017.414. Cell Death Dis. 2017. PMID: 28837139 Free PMC article.
-
Emodin mitigates podocytes apoptosis induced by endoplasmic reticulum stress through the inhibition of the PERK pathway in diabetic nephropathy.Drug Des Devel Ther. 2018 Jul 13;12:2195-2211. doi: 10.2147/DDDT.S167405. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30034224 Free PMC article.
-
Qiwei granules alleviates podocyte lesion in kidney of diabetic KK-Ay mice.BMC Complement Altern Med. 2015 Mar 31;15:97. doi: 10.1186/s12906-015-0603-x. BMC Complement Altern Med. 2015. PMID: 25887645 Free PMC article.
-
Stress in the kidney is the road to pERdition: is endoplasmic reticulum stress a pathogenic mediator of diabetic nephropathy?J Endocrinol. 2014 Sep;222(3):R97-111. doi: 10.1530/JOE-13-0517. Epub 2014 Jun 30. J Endocrinol. 2014. PMID: 24982467 Review.
-
[Pathomechanisms of podocyte injury in diabetic nephropathy and interventional effects of Chinese herbal medicine].Zhongguo Zhong Yao Za Zhi. 2016 Jul;41(13):2416-2421. doi: 10.4268/cjcmm20161308. Zhongguo Zhong Yao Za Zhi. 2016. PMID: 28905562 Review. Chinese.
Cited by
-
Apelin inhibited epithelial-mesenchymal transition of podocytes in diabetic mice through downregulating immunoproteasome subunits β5i.Cell Death Dis. 2018 Oct 9;9(10):1031. doi: 10.1038/s41419-018-1098-4. Cell Death Dis. 2018. PMID: 30301930 Free PMC article.
-
Exploring the Molecular Modalities in the Pathogenesis of Diabetic Kidney Disease with a Focus on the Potential Therapeutic Implications.Biomedicines. 2024 Dec 28;13(1):50. doi: 10.3390/biomedicines13010050. Biomedicines. 2024. PMID: 39857634 Free PMC article. Review.
-
Association between Renal Podocalyxin Expression and Renal Dysfunction in Patients with Diabetic Nephropathy: A Single-Center, Retrospective Case-Control Study.Biomed Res Int. 2020 Apr 3;2020:7350781. doi: 10.1155/2020/7350781. eCollection 2020. Biomed Res Int. 2020. PMID: 32337271 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. CVII. Structure and Pharmacology of the Apelin Receptor with a Recommendation that Elabela/Toddler Is a Second Endogenous Peptide Ligand.Pharmacol Rev. 2019 Oct;71(4):467-502. doi: 10.1124/pr.119.017533. Pharmacol Rev. 2019. PMID: 31492821 Free PMC article. Review.
-
Coregulation Analysis of Mechanistic Biomarkers in Autosomal Dominant Polycystic Kidney Disease.Int J Mol Sci. 2021 Jun 26;22(13):6885. doi: 10.3390/ijms22136885. Int J Mol Sci. 2021. PMID: 34206927 Free PMC article.
References
-
- Stieger N, Worthmann K, Schiffer M. The role of metabolic and haemodynamic factors in podocyte injury in diabetes. Diabetes Metab Res Rev. 2011;27:207–15. - PubMed
-
- Barisoni L. Podocyte biology in segmental sclerosis and progressive glomerular injury. Adv Chronic Kidney Dis. 2012;19:76–83. - PubMed
-
- Daroux M, Prevost G, Maillard-Lefebvre H, et al. Advanced glycation end-products: implications for diabetic and non-diabetic nephropathies. Diabetes Metab. 2010;36:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

