Grb2 monomer-dimer equilibrium determines normal versus oncogenic function
- PMID: 26103942
- PMCID: PMC4491180
- DOI: 10.1038/ncomms8354
Grb2 monomer-dimer equilibrium determines normal versus oncogenic function
Erratum in
-
Corrigendum: Grb2 monomer-dimer equilibrium determines normal versus oncogenic function.Nat Commun. 2015 Aug 3;6:8007. doi: 10.1038/ncomms9007. Nat Commun. 2015. PMID: 26234419 Free PMC article. No abstract available.
Abstract
The adaptor protein growth factor receptor-bound protein 2 (Grb2) is ubiquitously expressed in eukaryotic cells and involved in a multitude of intracellular protein interactions. Grb2 plays a pivotal role in tyrosine kinase-mediated signal transduction including linking receptor tyrosine kinases to the Ras/mitogen-activated protein (MAP) kinase pathway, which is implicated in oncogenic outcome. Grb2 exists in a constitutive equilibrium between monomeric and dimeric states. Here we show that only monomeric Grb2 is capable of binding to SOS and upregulating MAP kinase signalling and that the dimeric state is inhibitory to this process. Phosphorylation of tyrosine 160 (Y160) on Grb2, or binding of a tyrosylphosphate-containing ligand to the SH2 domain of Grb2, results in dimer dissociation. Phosphorylation of Y160 on Grb2 is readily detectable in the malignant forms of human prostate, colon and breast cancers. The self-association/dissociation of Grb2 represents a switch that regulates MAP kinase activity and hence controls cancer progression.
Figures

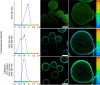
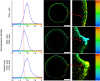
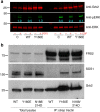

References
-
- Rozakis-Adcock M. et al. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature 360, 689–692 (1992). - PubMed
-
- Chu J., Liu Y., Koretzky G. A. & Durden D. L. SLP-76-Cbl-Grb2-Shc interactions in FcgammaRI signaling. Blood 92, 1697–1706 (1998). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

