Asymptomatic transmission and the resurgence of Bordetella pertussis
- PMID: 26103968
- PMCID: PMC4482312
- DOI: 10.1186/s12916-015-0382-8
Asymptomatic transmission and the resurgence of Bordetella pertussis
Abstract
Background: The recent increase in whooping cough incidence (primarily caused by Bordetella pertussis) presents a challenge to both public health practitioners and scientists trying to understand the mechanisms behind its resurgence. Three main hypotheses have been proposed to explain the resurgence: 1) waning of protective immunity from vaccination or natural infection over time, 2) evolution of B. pertussis to escape protective immunity, and 3) low vaccine coverage. Recent studies have suggested a fourth mechanism: asymptomatic transmission from individuals vaccinated with the currently used acellular B. pertussis vaccines.
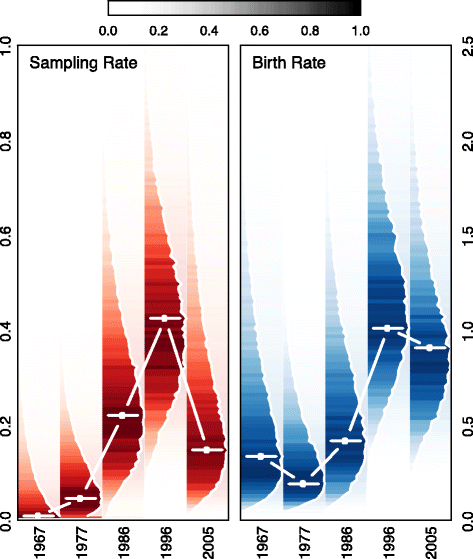
Methods: Using wavelet analyses of B. pertussis incidence in the United States (US) and United Kingdom (UK) and a phylodynamic analysis of 36 clinical B. pertussis isolates from the US, we find evidence in support of asymptomatic transmission of B. pertussis. Next, we examine the clinical, public health, and epidemiological consequences of asymptomatic B. pertussis transmission using a mathematical model.
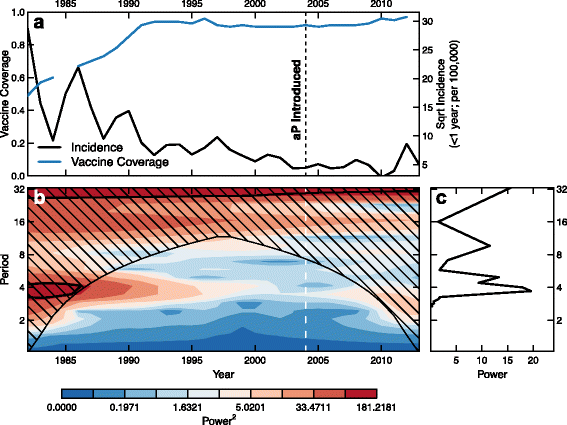
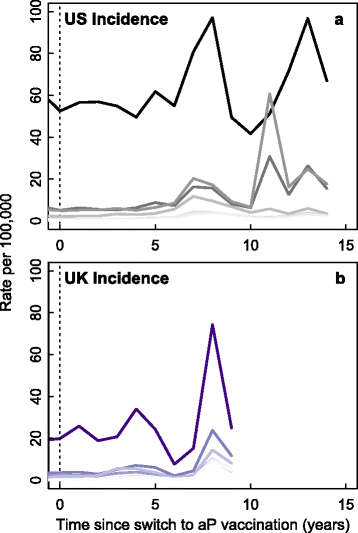
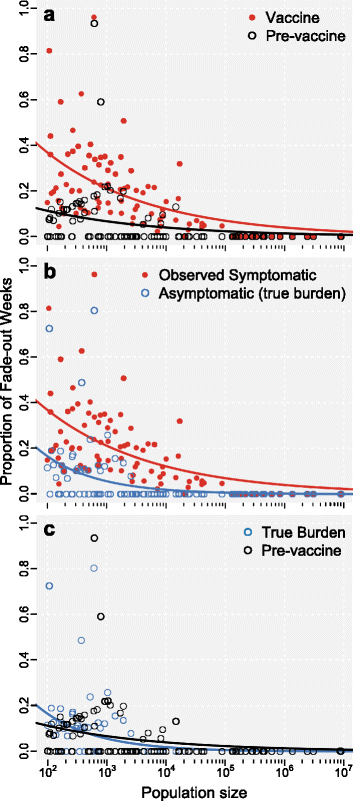
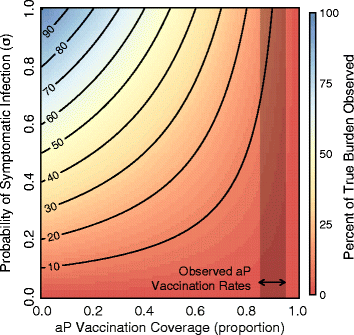
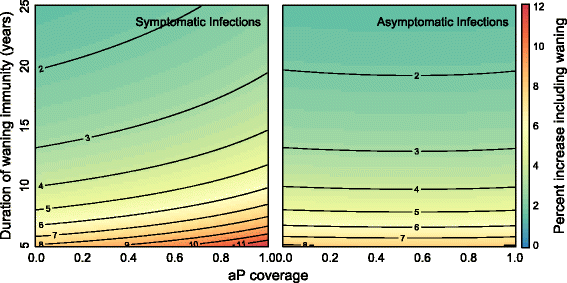
Results: We find that: 1) the timing of changes in age-specific attack rates observed in the US and UK are consistent with asymptomatic transmission; 2) the phylodynamic analysis of the US sequences indicates more genetic diversity in the overall bacterial population than would be suggested by the observed number of infections, a pattern expected with asymptomatic transmission; 3) asymptomatic infections can bias assessments of vaccine efficacy based on observations of B. pertussis-free weeks; 4) asymptomatic transmission can account for the observed increase in B. pertussis incidence; and 5) vaccinating individuals in close contact with infants too young to receive the vaccine ("cocooning" unvaccinated children) may be ineffective.
Conclusions: Although a clear role for the previously suggested mechanisms still exists, asymptomatic transmission is the most parsimonious explanation for many of the observations surrounding the resurgence of B. pertussis in the US and UK. These results have important implications for B. pertussis vaccination policy and present a complicated scenario for achieving herd immunity and B. pertussis eradication.
Figures



References
-
- CDC Pertussis (Whooping Cough) Surveillance & Reporting. http://www.cdc.gov/pertussis/surv-reporting.html.
-
- Office for National Statistics, UK, Datasets and reference tables. http://www.ons.gov.uk/ons/datasets-and-tables/index.html.
-
- World Health Organization. Progress Towards Global Immunization Goals - 2012. http://www.unicef.org/immunization/files/SlidesGlobalImmunization.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

