FAT1 cadherin acts upstream of Hippo signalling through TAZ to regulate neuronal differentiation
- PMID: 26104008
- PMCID: PMC11113810
- DOI: 10.1007/s00018-015-1955-6
FAT1 cadherin acts upstream of Hippo signalling through TAZ to regulate neuronal differentiation
Abstract
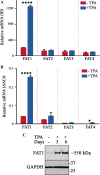
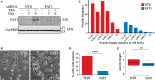
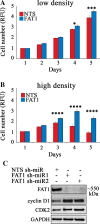
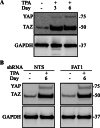
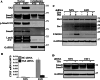
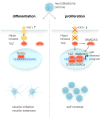
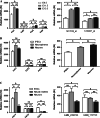
The Hippo pathway is emerging as a critical nexus that balances self-renewal of progenitors against differentiation; however, upstream elements in vertebrate Hippo signalling are poorly understood. High expression of Fat1 cadherin within the developing neuroepithelium and the manifestation of severe neurological phenotypes in Fat1-knockout mice suggest roles in neurogenesis. Using the SH-SY5Y model of neuronal differentiation and employing gene silencing techniques, we show that FAT1 acts to control neurite outgrowth, also driving cells towards terminal differentiation via inhibitory effects on proliferation. FAT1 actions were shown to be mediated through Hippo signalling where it activated core Hippo kinase components and antagonised functions of the Hippo effector TAZ. Suppression of FAT1 promoted the nucleocytoplasmic shuttling of TAZ leading to enhanced transcription of the Hippo target gene CTGF together with accompanying increases in nuclear levels of Smad3. Silencing of TAZ reversed the effects of FAT1 depletion thus connecting inactivation of TAZ-TGFbeta signalling with Hippo signalling mediated through FAT1. These findings establish FAT1 as a new upstream Hippo element regulating early stages of differentiation in neuronal cells.
Keywords: Cadherin; Differentiation; FAT1 cadherin; Hippo pathway; Neurite outgrowth; Neuronal differentiation; SMAD transcription factor; TAZ; TGFβ signalling.
Figures

References
-
- Dunne J, Hanby AM, Poulsom R, Jones TA, Sheer D, Chin WG, Da SM, Zhao Q, Beverley PC, Owen MJ. Molecular cloning and tissue expression of FAT, the human homologue of the Drosophila fat gene that is located on chromosome 4q34-q35 and encodes a putative adhesion molecule. Genomics. 1995;30(2):207–223. doi: 10.1006/geno.1995.9884. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

